A preregistered meta-meta-analysis on the global distribution of Hepatotropic Viruses
- Authors: Adeiza S.1,2, Islam M.3,4, Mungadi H.5, Shuaibu A.5, Sah R.6,7
-
Affiliations:
- Ahmadu Bello University
- Usmanu Dafodiyo University
- President Abdul Hamid Medical College
- Noakhali Science and Technology University
- Usmanu DanFodiyo University
- Institute of Medicine
- Dr. D. Y. Patil Medical College, Hospital and Research Centre, Dr. D.Y. Patil Vidyapeeth
- Issue: Vol 69, No 5 (2024)
- Pages: 429-440
- Section: ORIGINAL RESEARCHES
- URL: https://journal-vniispk.ru/0507-4088/article/view/269831
- DOI: https://doi.org/10.36233/0507-4088-234
- EDN: https://elibrary.ru/txidjt
- ID: 269831
Cite item
Full Text
Abstract
Introduction. Hepatotropic viruses (HAV, HBV, HCV, HDV, and HEV) significantly impact global health, with varying prevalence across regions.
Objective. This study aims to systematically consolidate data from diverse meta-analyses to provide a contemporary reference on virus distribution and prevalence.
Materials and methods. Adhering to PRISMA guidelines, the study utilized a mixed effects model for data integration. Quality evaluation was carried out with QUOROM and AMSTAR tools, with heterogeneity assessed via the Higgins I2 statistic, Q-statistic and Tau squared (τ2) values.
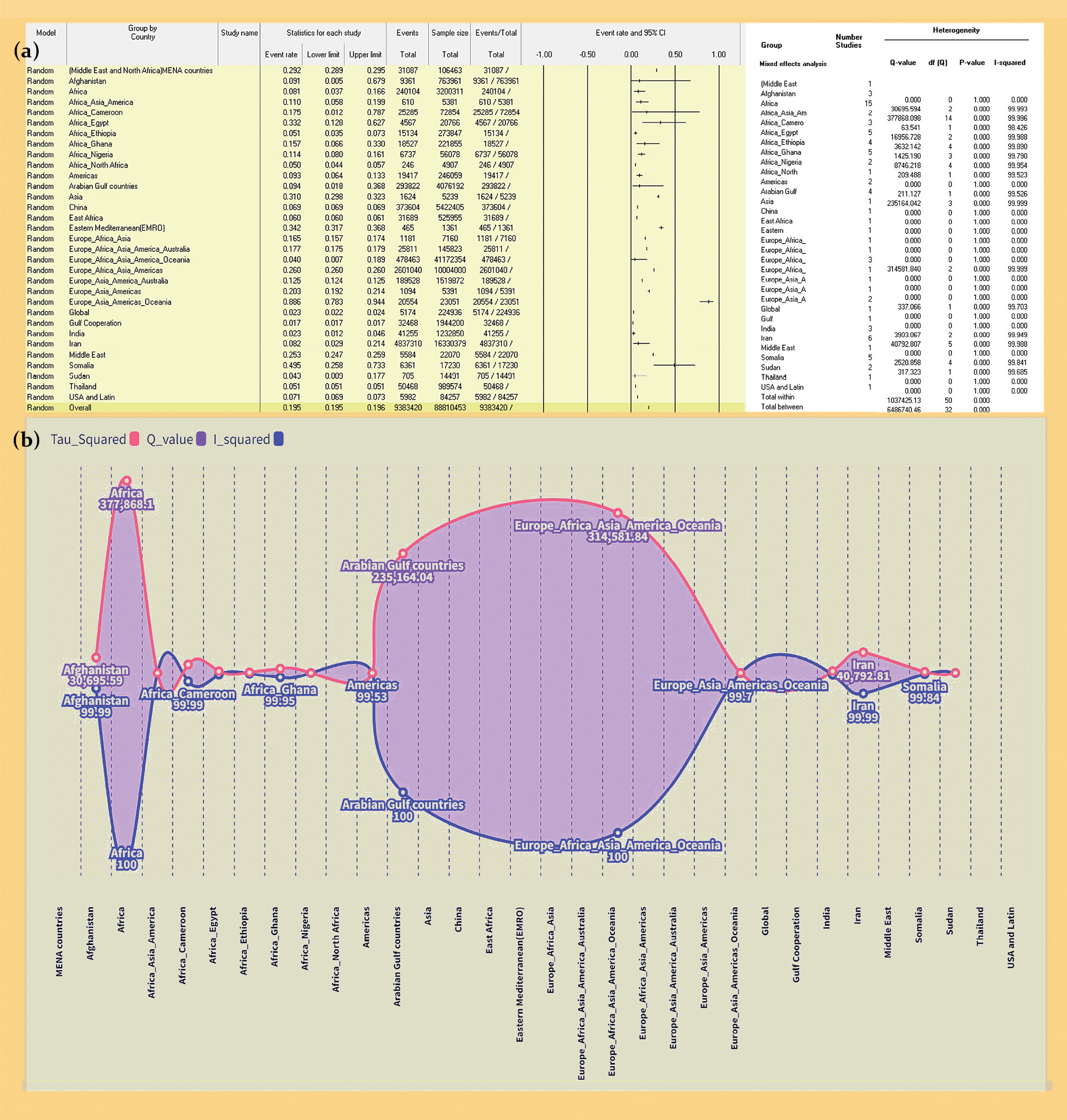
Results. The study analyzed 86 meta-analyses from 56 studies (2017–2022) with minimal overlap. Prevalence rates by region were as follows: MENA – 29.2%, Afghanistan – 9.14%, Africa – 8.10%. Prevalence rates by virus type: HAV – 82.5%, HBV – 8.6%, HCV – 15.1%, HDV – 8.9%, HEV – 13.9%, dual HBV-HCV coinfection – 2.2%. Prevalence rates by risk groups: general population – 8.3%, healthcare workers – 4.0%. Continent-specific HBV-HCV prevalence rates: Africa – 9.2%, China – 6.9%, others. HCVprevalence rates among at-risk groups: healthcare workers – 5.58%, hemodialysis patients – 34.8%. Regional HCV rates: Africa – 7.42%, Middle East – 25.30%.
Conclusion. Diverse global hepatotropic virus prevalence patterns are influenced by multifaceted factors. MENA faces higher rates due to healthcare challenges, while Africa struggles with limited resources. Tailored public health strategies, including vaccination and awareness campaigns, are essential to alleviate burdens and enhance global health. This consolidated data serves as a valuable resource for informed decision-making.
Keywords
Full Text
##article.viewOnOriginalSite##About the authors
Shuaibu Suleiman Adeiza
Ahmadu Bello University; Usmanu Dafodiyo University
Author for correspondence.
Email: suleykestler2@gmail.com
ORCID iD: 0000-0002-9293-2600
PhD, Head of Clinical Practice Laboratory, Department of Pharmaceutical Microbiology & Biotechnology, Faculty of Pharmaceutical Sciences, Department of Clinical Pharmacy and Pharmacy Practice, Faculty of Pharmaceutical Sciences
Nigeria, Zaria; SokotoMd. Aminul Islam
President Abdul Hamid Medical College; Noakhali Science and Technology University
Email: aminul@pahmc.edu.bd
ORCID iD: 0000-0003-1091-9726
MSc, PhD Scholar of Cell and Molecular Biology, Advanced Molecular Lab, Department of Microbiology, President, COVID-19 Diagnostic Lab, Department of Microbiology
Bangladesh, Karimganj, Kishoreganj, 2310; Noakhali, 3814Hauwa’u Umar Mungadi
Usmanu DanFodiyo University
Email: hauwaumarmng@gmail.com
ORCID iD: 0000-0001-7200-1035
PhD, Head of Department, Department of Veterinary Medicine, Faculty of Veterinary Medicine
Nigeria, SokotoAbdulmalik Bello Shuaibu
Usmanu DanFodiyo University
Email: abdulmalik.shuaibu@udusok.edu.ng
ORCID iD: 0000-0001-7684-2472
PhD (student), Head of Laboratory Virology Unit, Department of Veterinary Microbiology, Faculty of Veterinary Medicine
Nigeria, SokotoRanjit Sah
Institute of Medicine; Dr. D. Y. Patil Medical College, Hospital and Research Centre, Dr. D.Y. Patil Vidyapeeth
Email: ranjitsah@iom.edu.np
ORCID iD: 0000-0002-2695-8714
PhD, Head of Influenza and COVID-19 Testing (National Influenza Center), Department of Microbiology, Tribhuvan University Teaching Hospital, Department of Microbiology
Nepal, Kathmandu 44600; Pune, 411018, Maharashtra, IndiaReferences
- Lopez-Scarim J., Nambiar S.M., Billerbeck E. Studying T cell responses to hepatotropic viruses in the liver microenvironment. Vaccines (Basel). 2023; 11(3): 681. https://doi.org/10.3390/vaccines11030681
- Devarbhavi H., Asrani S.K., Arab J.P., Nartey Y.A., Pose E., Kamath P.S. Global burden of liver disease: 2023 update. J. Hepatology. 2023; 79(2): 516–37. https://doi.org/10.1016/j.jhep.2023.03.017
- Hassnine A.A., Saber M.A., Fouad Y.M., Sarhan H., Elsayed M.M., Zaki Z.M., et al. Clinical study on the efficacy of hepatitis B vaccination in hepatitis C virus related chronic liver diseases in Egypt. Virus Res. 2023; 323: 198953. https://doi.org/10.1016/j.virusres.2022.198953
- Antoniou T., Pritlove C., Shearer D., Tadrous M., Shah H., Gomes T. Accessing hepatitis C direct acting antivirals among people living with hepatitis C: a qualitative study. Int. J. Equity Health. 2023; 22(1): 112. https://doi.org/10.1186/s12939-023-01924-4
- Abesig J., Chen Y., Wang H., Sompo F.M., Wu I.X.Y. Prevalence of viral hepatitis B in Ghana between 2015 and 2019: A systematic review and meta-analysis. PLoS One. 2020; 15(6): e0234348. https://doi.org/10.1371/journal.pone.0234348
- Adane T., Getawa S. The prevalence and associated factors of hepatitis B and C virus in hemodialysis patients in Africa: A systematic review and meta-analysis. PLoS One. 2021; 16(6): e0251570. https://doi.org/10.1371/journal.pone.0251570
- Alali A.A., Abo-Shehada M.N. Prevalence of Hepatitis B Virus infection in the Gulf Cooperation Council: a systematic review and meta-analysis. BMC Infect. Dis. 2022; 22(1): 819. https://doi.org/10.1186/s12879-022-07806-4
- Kenfack-Momo R., Kenmoe S., Takuissu G.R., Ebogo-Belobo J.T., Kengne-Ndé C., Mbaga D.S., et al. Epidemiology of hepatitis B virus and/or hepatitis C virus infections among people living with human immunodeficiency virus in Africa: A systematic review and meta-analysis. PLoS One. 2022; 17(5): e0269250. https://doi.org/10.1371/journal.pone.0269250
- Qashqari F.S. Seroprevalence of hepatitis E virus infection in Middle Eastern Countries: A systematic review and meta-analysis. Medicina (Kaunas). 2022; 58(7): 905. https://doi.org/10.3390/medicina58070905
- Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol. Hepatol. 2022; 7(5): 396–415. https://doi.org/10.1016/s2468-1253(21)00472-6
- Irekeola A.A., Ear E.N.S., Mohd Amin N.A.Z., Mustaffa N., Shueb R.H. Antivirals against HCV infection: the story thus far. J. Infect. Dev. Ctries. 2022; 16(2): 231–43. https://doi.org/10.3855/jidc.14485
- Adeiza SS, Aminul I. Meta-meta-analysis of the mortality risk associated with MRSA compared to MSSA bacteraemia. Infez. Med. 2024;32(2):131. http://dx.doi.org/10.53854/liim-3202-2
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 2021; 10(1): 89. https://doi.org/10.1186/s13643-021-01626-4
- Christensen E. Quality of reporting of meta-analyses: the QUOROM statement. Will it help? J. Hepatol. 2001; 34(2): 342–5. https://doi.org/10.1016/s0168-8278(00)00002-7
- Suleiman A.S., Islam M.A., Akter M.S., Amin M.R., Werkneh A.A., Bhattacharya P. A meta-meta-analysis of co-infection, secondary infections, and antimicrobial resistance in COVID-19 patients. J. Infect. Public Health. 2023; 16(10): 1562–90. https://doi.org/10.1016/j.jiph.2023.07.005
- Shea B.J., Hamel C., Wells G.A., Bouter L.M., Kristjansson E., Grimshaw J., et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J. Clin. Epidemiol. 2009; 62(10): 1013–20. https://doi.org/10.1016/j.jclinepi.2008.10.009
- Adeiza S.S., Shuaibu A.B., Shuaibu G.M. Random effects meta-analysis of COVID-19/S. aureus partnership in co-infection. GMS Hyg. Infect. Control. 2020; 15: Doc29. https://doi.org/10.3205/dgkh000364
- Adeiza SS, Islam MA, Shittu A. Global, regional, and national burdens: An overlapping meta-analysis on Staphylococcus aureus and its drug-resistant strains. One Health Bull. 2024:10-4103. https://doi.org/10.4103/ohbl.ohbl_10_24
- Borenstein M. In a meta-analysis, the I-squared statistic does not tell us how much the effect size varies. J. Clin. Epidemiol. 2022; 152: 281–4. https://doi.org/10.1016/j.jclinepi.2022.10.003
- Borenstein M. Comprehensive meta-analysis software. In: Systematic Reviews in Health Research: Meta-Analysis in Context. Wiley; 2022: 535–48.
- Agyeman A.A., Ofori-Asenso R. Prevalence of HIV and hepatitis B coinfection in Ghana: a systematic review and meta-analysis. AIDS Res Ther. 2016; 13: 23. https://doi.org/10.1186/s12981-016-0107-x
- Agyeman A.A., Ofori-Asenso R., Mprah A., Ashiagbor G. Epidemiology of hepatitis C virus in Ghana: a systematic review and meta-analysis. BMC Infect. Dis. 2016; 16: 391. https://doi.org/10.1186/s12879-016-1708-7
- Ahmad T., Hui J., Musa T.H., Behzadifar M., Baig M. Seroprevalence of hepatitis E virus infection in pregnant women: a systematic review and meta-analysis. Ann. Saudi Med. 2020; 40(2): 136–46. https://doi.org/10.5144/0256-4947.2020.136
- Ajuwon B.I., Yujuico I., Roper K., Richardson A., Sheel M., Lidbury B.A. Hepatitis B virus infection in Nigeria: a systematic review and meta-analysis of data published between 2010 and 2019. BMC Infect. Dis. 2021; 21(1): 1120. https://doi.org/10.1186/s12879-021-06800-6
- Alemu A.A., Zeleke L.B., Aynalem B.Y., Kassa G.M. Hepatitis B virus infection and its determinants among pregnant women in Ethiopia: a systematic review and meta-analysis. Infect. Dis. Obstet. Gynecol. 2020; 2020: 9418475. https://doi.org/10.1155/2020/9418475
- Alonso M., Gutzman A., Mazin R., Pinzon C.E., Reveiz L., Ghidinelli M. Hepatitis C in key populations in Latin America and the Caribbean: systematic review and meta-analysis. Int. J. Public Health. 2015; 60(7): 789–98. https://doi.org/10.1007/s00038-015-0708-5
- Amini N., Alavian S.M., Kabir A., Aalaei-Andabili S.H., Saiedi Hosseini S.Y., Rizzetto M. Prevalence of hepatitis D in the Eastern Mediterranean region: systematic review and meta-analysis. Hepat. Mon. 2013; 13(1): e8210. https://doi.org/10.5812/hepatmon.8210
- Ashkani-Esfahani S., Alavian S.M., Salehi-Marzijarani M. Prevalence of hepatitis C virus infection among hemodialysis patients in the Middle-East: A systematic review and meta-analysis. World J. Gastroenterol. 2017; 23(1): 151. https://doi.org/10.3748/wjg.v23.i1.151
- Atlaw D., Sahiledengle B., Tariku Z. Hepatitis B and C virus infection among healthcare workers in Africa: a systematic review and meta-analysis. Environ. Health Prev. Med. 2021; 26(1): 61. https://doi.org/10.1186/s12199-021-00983-9
- Azami M., Hafezi Ahmadi M.R., Sayehmiri K. Hepatitis B vaccination efficacy in Iranian healthcare workers: a meta-analysis study. Hepat. Mon. 2017; 17(1): e37781. https://doi.org/10.5812/hepatmon.37781
- Badawi M.M., Atif M.S., Mustafa Y.Y. Systematic review and meta-analysis of HIV, HBV and HCV infection prevalence in Sudan. Virol. J. 2018; 15(1): 148. https://doi.org/10.1186/s12985-018-1060-1
- Batham A., Narula D., Toteja T., Sreenivas V., Puliyel J.M. Systematic review and meta-analysis of prevalence of hepatitis B in India. Indian Pediatrics. 2007; 44(9): 663.
- Chemaitelly H., Mahmud S., Rahmani A.M., Abu-Raddad L.J. The epidemiology of hepatitis C virus in Afghanistan: systematic review and meta-analysis. Int. J. Infect. Dis. 2015; 40: 54–63. https://doi.org/10.1016/j.ijid.2015.09.011
- Chen H.Y., Shen D.T., Ji D.Z., Han P.C., Zhang W.M., Ma J.F., et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2019; 68(3): 512–21. https://doi.org/10.1136/gutjnl-2018-316601
- Daw M.A., Daw A.M., Sifennasr N.E.M., Draha A.M., Daw A.M., Daw A.M., et al. The epidemiology of hepatitis D virus in North Africa: a systematic review and meta-analysis. ScientificWorldJournal. 2018; 2018: 9312650. https://doi.org/10.1155/2018/9312650
- Desikan P., Khan Z. Prevalence of hepatitis B and hepatitis C virus co-infection in India: a systematic review and meta-analysis. Indian J. Med. Microbiol. 2017; 35(3): 332–9. https://doi.org/10.4103/ijmm.ijmm_17_257
- Fernández Villalobos N.V., Kessel B., Rodiah I., Ott J.J., Lange B., Krause G. Seroprevalence of hepatitis E virus infection in the Americas: Estimates from a systematic review and meta-analysis. PLoS One. 2022; 17(6): e0269253. https://doi.org/10.1371/journal.pone.0269253
- Farajzadegan Z., Hoseini S.G., Kelishadi R., Jamshidi F., Nokhodian Z., Noori R., et al. Systematic review and meta-analysis on the age-specific seroprevalence of hepatitis A in Iran. J. Res. Med. Sci. 2014; 19(Suppl. 1): S56–63.
- Giri S., Sahoo S., Angadi S., Afzalpurkar S., Sundaram S., Bhrugumalla S. Seroprevalence of hepatitis B virus among pregnant women in India: A systematic review and meta-analysis. J. Clin. Exp. Hepatol. 2022; 12(6): 1408–19. https://doi.org/10.1016/j.jceh.2022.08.005
- Harfouche M., Chemaitelly H., Mahmud S., Chaabna K., Kouyoumjian S.P., Al Kanaani Z., et al. Epidemiology of hepatitis C virus among hemodialysis patients in the Middle East and North Africa: systematic syntheses, meta-analyses, and meta-regressions. Epidemiol. Infect. 2017; 145(15): 3243–63. https://doi.org/10.1017/s0950268817002242
- Hassan-Kadle M.A., Osman M.S., Ogurtsov P.P. Epidemiology of viral hepatitis in Somalia: Systematic review and meta-analysis study. World J. Gastroenterol. 2018; 24(34): 3927–57. https://doi.org/10.3748/wjg.v24.i34.3927
- Horvatits T., Ozga A.K., Westhölter D., Hartl J., Manthey C.F., Lütgehetmann M., et al. Hepatitis E seroprevalence in the Americas: A systematic review and meta-analysis. Liver Int. 2018; 38(11): 1951–64. https://doi.org/10.1111/liv.13859
- Hughes E., Bassi S., Gilbody S., Bland M., Martin F., et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness: a systematic review and meta-analysis. Lancet Psychiatry. 2016; 3(1): 40–8. https://doi.org/10.1016/s2215-0366(15)00357-0
- Kafeero H.M., Ndagire D., Ocama P., et al. Prevalence and predictors of hepatitis B virus (HBV) infection in east Africa: evidence from a systematic review and meta-analysis of epidemiological studies published from 2005 to 2020. Arch. Public Health. 2021; 79(1): 167. https://doi.org/10.1186/s13690-021-00686-1
- Kouyoumjian S.P., Chemaitelly H., Abu-Raddad L.J. Characterizing hepatitis C virus epidemiology in Egypt: systematic reviews, meta-analyses, and meta-regressions. Sci. Rep. 2018; 8(1): 1661. https://doi.org/10.1038/s41598-017-17936-4
- Larney S., Kopinski H., Beckwith C.G., Zaller N.D., Jarlais D.D., Hagan H., et al. Incidence and prevalence of hepatitis C in prisons and other closed settings: Results of a systematic review and meta-analysis. Hepatology. 2013; 58(4): 1215–24. https://doi.org/10.1002/hep.26387
- Leroi C., Adam P., Khamduang W., Kawilapat S., Ngo-Giang-Huong N., Ongwandee S., et al. Prevalence of chronic hepatitis B virus infection in Thailand: a systematic review and meta-analysis. Int. J. Infect. Dis. 2016; 51: 36–43. https://doi.org/10.1016/j.ijid.2016.08.017
- Leumi S., Bigna J.J., Amougou M.A., Ngouo A., Nyaga U.F., Noubiap J.J. Global burden of hepatitis B infection in people living with human immunodeficiency virus: a systematic review and meta-analysis. Clin. Infect. Dis. 2020; 71(11): 2799–806. https://doi.org/10.1093/cid/ciz1170
- Li P., Liu J., Li Y., Su J., Ma Z., Bramer W.M., et al. The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int. 2020; 40(7): 1516–28. https://doi.org/10.1111/liv.14468
- Mahamat G., Kenmoe S., Akazong E.W., Ebogo-Belobo J.T., Mbaga D.S., Bowo-Ngandji A., et al. Global prevalence of hepatitis B virus serological markers among healthcare workers: A systematic review and meta-analysis. World J. Hepatol. 2021; 13(9): 1190–202. https://doi.org/10.4254/wjh.v13.i9.1190
- Mahmud S., Akbarzadeh V., Abu-Raddad L.J. The epidemiology of hepatitis C virus in Iran: Systematic review and meta-analyses. Sci. Rep. 2018; 8(1): 150. https://doi.org/10.1038/s41598-017-18296-9
- Moghaddasifar I, B. Lankarani K, Moosazadeh M, et al. Prevalence of hepatitis B virus infection among pregnant women in Iran: A systematic review and meta-analysis. Iran. J. Cancer Prev. 2016; 9(6). https://doi.org/10.17795/ijcp-3703
- Mohamoud Y.A., Riome S., Abu-Raddad L.J. Epidemiology of hepatitis C virus in the Arabian Gulf countries: Systematic review and meta-analysis of prevalence. Int. J. Infect. Dis. 2016; 46: 116–25. https://doi.org/10.1016/j.ijid.2016.03.012
- Mora N., Adams W.H., Kliethermes S., Dugas L., Balasubramanian N., Sandhu J., et al. A synthesis of hepatitis C prevalence estimates in Sub-Saharan Africa: 2000–2013. BMC Infect. Dis. 2016; 16: 283. https://doi.org/10.1186/s12879-016-1584-1
- Musa B., Bussell S., Borodo M., Samaila A.A., Femi O.L. Prevalence of hepatitis B virus infection in Nigeria, 2000-2013: A systematic review and meta-analysis. Niger. J. Clin. Pract. 2015; 18(2): 163–72. https://doi.org/10.4103/1119-3077.151035
- Ofori-Asenso R., Agyeman A.A. Hepatitis B in Ghana: a systematic review & meta-analysis of prevalence studies (1995–2015). BMC Infect. Dis. 2016; 16: 130. https://doi.org/10.1186/s12879-016-1467-5
- Rao V.B., Johari N., Du Cros P., Messina J., Ford N., Cooke G.S. Hepatitis C seroprevalence and HIV co-infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect. Dis. 2015; 15(7): 819–24. https://doi.org/10.1016/s1473-3099(15)00006-7
- Rossi C., Shrier I., Marshall L., Cnossen S., Schwartzman K., Klein M.B., et al. Seroprevalence of chronic hepatitis B virus infection and prior immunity in immigrants and refugees: a systematic review and meta-analysis. PLoS One. 2012; 7(9): e44611. https://doi.org/10.1371/journal.pone.0044611
- Salari N., Darvishi N., Hemmati M., Shohaimi S., Ghyasi Y., Hossaini F., et al. Global prevalence of hepatitis C in prisoners: a comprehensive systematic review and meta-analysis. Arch. Virol. 2022; 167(4): 1025–39. https://doi.org/10.1007/s00705-022-05382-1
- Salehi-Vaziri M., Sadeghi F., Almasi Hashiani A., Gholami Fesharaki M., Alavian S.M. Hepatitis B virus infection in the general population of Iran: an updated systematic review and meta-analysis. Hepat. Mon. 2016; 16(4): e35577. https://doi.org/10.5812/hepatmon.35577
- Souza-Silva G., Zolnikov T.R., Ortolani P.L., Cruvinel V.R.N., Dias S.M., Mol M.P.G. Hepatitis B and C prevalence in waste pickers: a global meta-analysis. J. Public Health (Oxf.). 2022; 44(4): 761–9. https://doi.org/10.1093/pubmed/fdab285
- Stockdale A.J., Kreuels B., Henrion M.Y.R., Giorgi E., Kyomuhangi I., de Martel C., et al. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J. Hepatol. 2020; 73(3): 523–32. https://doi.org/10.1016/j.jhep.2020.04.008
- Wang H., Men P., Xiao Y., Gao P., Lv M., Yuan Q., et al. Hepatitis B infection in the general population of China: a systematic review and meta-analysis. BMC Infect. Dis. 2019; 19(1): 811. https://doi.org/10.1186/s12879-019-4428-y
- Yazie T.D., Tebeje M.G. An updated systematic review and meta-analysis of the prevalence of hepatitis B virus in Ethiopia. BMC Infect. Dis. 2019; 19(1): 917. https://doi.org/10.1186/s12879-019-4486-1
- Azevedo T.C.L., Zwahlen M., Rauch A., Egger M., Wandeler G. Hepatitis C in HIV-infected individuals: a systematic review and meta-analysis of estimated prevalence in Africa. J. Int. AIDS Soc. 2016; 19(1): 20711. https://doi.org/10.7448/ias.19.1.20711
- Li P., Ji Y., Li Y., Ma Z., Pan Q. Estimating the global prevalence of hepatitis E virus in swine and pork products. One Health. 2022; 14: 100362. https://doi.org/10.1016/j.onehlt.2021.100362
- Takuissu G.R., Kenmoe S., Amougou Atsama M., Atenguena Okobalemba E., Mbaga D.S., Ebogo-Belobo J.T., et al. Global epidemiology of occult hepatitis B virus infections in blood donors, a systematic review and meta-analysis. PLoS One. 2022; 17(8): e0272920. https://doi.org/10.1371/journal.pone.0272920
- Hofmeister M.G., Xing J., Foster M.A., Augustine R.J., Burkholder C., Collins J., et al. Hepatitis A person-to-person outbreaks: epidemiology, morbidity burden, and factors associated with hospitalization—multiple states, 2016–2019. J. Infect. Dis. 2021; 223(3): 426–34. https://doi.org/10.1093/infdis/jiaa636
- Olaru I.D., Beliz Meier M., Mirzayev F., Prodanovic N., Kitchen P.J., Schumacher S.G., et al. Global prevalence of hepatitis B or hepatitis C infection among patients with tuberculosis disease: systematic review and meta-analysis. EClinicalMedicine. 2023; 58: 101938. https://doi.org/10.1016/j.eclinm.2023.101938
- Makokha G.N., Zhang P., Hayes C.N., Songok E., Chayama K. The burden of Hepatitis B virus infection in Kenya: A systematic review and meta-analysis. Front. Public Health. 2023; 11: 986020. https://doi.org/10.3389/fpubh.2023.986020
- Yendewa G.A., Wang G.M., James P.B., Massaquoi S.P.E., Yendewa S.A., Ghazawi M., et al. Prevalence of chronic hepatitis B virus infection in Sierra Leone, 1997–2022: A systematic review and meta-analysis. Am. J. Trop. Med. Hyg. 2023; 109(1): 105–14. https://doi.org/10.4269/ajtmh.22-0711
- Qiu L.X., Huang Y., Quan J.L., Bi Z.F., Zhong G.H., Wang J.Y., et al. Prognosis of hepatitis E infection in patients with chronic liver disease: A meta-analysis. J. Viral Hepat. 2023; 30(2): 101–7. https://doi.org/10.1111/jvh.13754
- Raji Y.E., Toung O.P., Taib N.M., Sekawi Z.B. Meta-analysis and moderator analysis of the seroprevalence of hepatitis E in South-Eastern Asia. Sci. Rep. 2023; 13(1): 11880. https://doi.org/10.1038/s41598-023-37941-0
- Asselah T., Rizzetto M. Hepatitis D virus infection. N. Engl. J. Med. 2023; 389(1): 58–70. https://doi.org/10.1056/nejmra2212151
- Magri M.C., Manchiero C., Dantas B.P., da Silva Nunes A.K., Vaz Gago Prata T., Domingos D.E.A., et al. Hepatitis C among people who inject drugs (PWID) in Latin America and the Caribbean: A meta-analysis of prevalence over three decades. J. Stud. Alcohol Drugs. 2023; 84(1): 118–27. https://doi.org/10.15288/jsad.22-00014
- Vincent J.P., Nyamasege C., Wang S., Madec Y., Shimakawa Y. Prevalence of hepatitis B, C, and D virus infection in Haiti: A systematic review and meta-analysis. Front. Public Health. 2023; 10: 1099571. https://doi.org/10.3389/fpubh.2022.1099571
- Aghaei A.M., Gholami J., Sangchooli A., Rostam-Abadi Y., Olamazadeh S., Ardeshir M., et al. Prevalence of injecting drug use and HIV, hepatitis B, and hepatitis C in people who inject drugs in the Eastern Mediterranean region: a systematic review and meta-analysis. Lancet Glob Health. 2023; 11(8): e1225–37. https://doi.org/10.1016/s2214-109x(23)00267-x
Supplementary files