Interferon-regulating activity of the CelAgrip drug and its influence on the formation of reactive oxygen species and expression of innate immunity genes in Burkitt’s lymphome cell cultures
- Authors: Narovlyansky A.N.1, Poloskov V.V.2, Ivanova A.M.2, Mezentseva M.V.2, Suetina I.A.2, Russu L.I.2, Chelarskaya E.S.2, Izmest’Eva A.V.2, Ospelnikova T.P.2, Zubashev I.K.2, Sarymsakov A.A.3, Ershov F.I.2
-
Affiliations:
- Doctor of Biological Sciences, Chief Researcher of the Cytokine Laboratory, National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya, Moscow, 123098, Russia
- National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya
- Institute of Polymer Chemystry and Physics
- Issue: Vol 65, No 2 (2020)
- Pages: 87-94
- Section: ORIGINAL RESEARCHES
- URL: https://journal-vniispk.ru/0507-4088/article/view/118032
- DOI: https://doi.org/10.36233/0507-4088-2020-65-2-87-94
- ID: 118032
Cite item
Full Text
Abstract
Introduction. Interferons (IFN) and IFN inducers are effective in suppressing viral reproduction and correcting of the innate immunity mechanisms.
The aim of the study was to test the hypothesis of the possible involvement of the IFN inducer CelAgrip (CA) as an activator or suppressor of antiviral effects in Burkitt’s lymphoma (LB) cell cultures with different ability to produce Epstein-Barr virus antigens (EBV).
Material and methods. The kinetic analysis of the dynamics of reactive oxygen species (ROS) production and determination of gene group expression by real-time PCR in response to CA treatment were done in human cell lines LB P3HR-1 and Namalva, spontaneously producing and not producing EBV antigens.
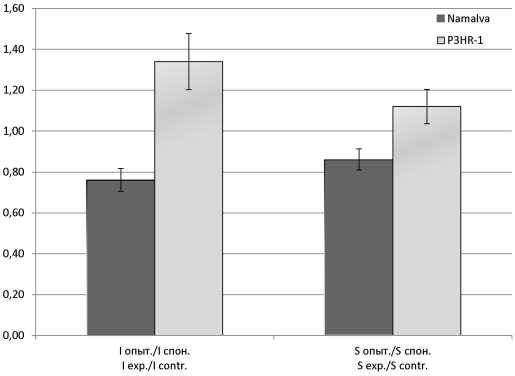
Results and discussion. When treating CA in Namalva cells, a decrease in the ROS activation index was found; in P3HR-1 cells, an increase was observed. After treatment with CA, there was no reliable activation of the IFN-α, IFN-β and IFN-λ genes in Namalva cells, but the expression of the ISG15 and P53(TP53) genes was increased more than 1200 times and 4.5 times, respectively. When processing the CA of P3HR-1 cells, the expression of IFN-α genes increased by more than 200 times, IFN-λ - 100 times, and the ISG15 gene - 2.2 times. The relationship between IFN-inducing action of CA and the activity of ISG15 and ROS in LB cell cultures producing and not producing EBV antigens is supposed.
Conclusion. In Namalva cells that do not produce EBV antigens the treatment of CA results in suppression of ROS generation and activation of the expression of genes ISG15 and P53 (TP53); in P3HR-1 cells producing EBV antigens, the opposite picture is observed - the formation of ROS and the expression of the IFN-α and IFN-λ genes are activated and the activity of the ISG15 and P53 (TP53) genes is suppressed.
Full Text
##article.viewOnOriginalSite##About the authors
Alexander N. Narovlyansky
Doctor of Biological Sciences, Chief Researcher of the Cytokine Laboratory, National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya, Moscow, 123098, Russia
Author for correspondence.
Email: narovl@yandex.ru
ORCID iD: 0000-0003-0601-7148
Vladislav V. Poloskov
National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya
ORCID iD: 0000-0003-0001-2493
Russian Federation
Alla M. Ivanova
National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya
ORCID iD: 0000-0002-6008-7967
Russian Federation
Marina V. Mezentseva
National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya
ORCID iD: 0000-0001-7346-5536
Russian Federation
Irina A. Suetina
National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya
ORCID iD: 0000-0003-2878-0590
Russian Federation
Leonid I. Russu
National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya
ORCID iD: 0000-0001-6353-9917
Russian Federation
Ekaterina S. Chelarskaya
National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya
ORCID iD: 0000-0002-5254-0493
Russian Federation
Anna V. Izmest’Eva
National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya
ORCID iD: 0000-0002-0035-324X
Russian Federation
Tatiyana P. Ospelnikova
National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya
ORCID iD: 0000-0002-1580-6096
Russian Federation
Igor K. Zubashev
National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya
ORCID iD: 0000-0002-3238-7778
Russian Federation
Abdushukur A. Sarymsakov
Institute of Polymer Chemystry and Physics
ORCID iD: 0000-0003-4562-7280
Russian Federation
Feliks I. Ershov
National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya
ORCID iD: 0000-0002-4780-7560
Russian Federation
References
- Ершов Ф.И., Киселев О.И. Интерфероны и их индукторы (от молекул до лекарств). М.: ГЭОТАР-Медиа; 2005.
- Ершов Ф.И., Наровлянский А.Н. Интерфероны и индукторы интерферонов. В кн.: Хаитов Р.М., Атауллаханов Р.И., Шульженко А.Е., ред. Иммунотерапия: руководство для врачей. М.: ГЭОТАР-Медиа; 2018: 123-47.
- Segal A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005; 23: 197-223. DOI: http://doi.org/10.1146/annurev.immunol.23.021704.115653
- Зайцев В.Г., Закревский В.И. Методологические аспекты исследований свободно-радикального окисления и антиоксидантной системы организма. Вестник Волгоградской медицинской академии: Сборник научных трудов. 1998; 54(4): 49-53.
- Cross A.R., Jones O.T.G. Enzymic mechanism of superoxide production. Biochem. Biophys. Acta. 1991; 1057(3): 281-98. DOI: http://doi.org/10.1016/S0005-2728(05)80140-9
- Sandhu S.K., Kaur G. Mitochondrial electron transport chain complexes in aging rat brain and lymphocytes. Biogerontol. 2003; 4(1): 19-29. DOI: http://doi.org/10.1023/a:1022473219044
- Донцов В.И., Крутько В.Н., Мрикаев Б.М., Уханов С.В. Активные формы кислорода как система: значение в физиологии, патологии и естественном старении. Труды Института системного анализа Российской академии наук. 2006; 19: 50-69.
- Новиков В.Е., Левченкова О.С., Пожилова Е.В. Роль активных форм кислорода в физиологии и патологии клетки и их фармакологическая регуляция. Обзоры по клинической фармакологии и лекарственной терапии. 2014; 12(4): 13-21.
- Jha H.C., Pei Y., Robertson E.S. Epstein-Barr virus: Diseases linked to infection and transformation. Front. Microbiol. 2016; 7: 1602. DOI: http://doi.org/10.3389/fmicb.2016.01602
- Ascherio A., Munger K.L. EBV and autoimmunity. Curr. Top. Microbiol. Immunol. 2015; 390(Pt. 1): 365-85. DOI: http://doi.org/10.1007/978-3-319-22822-8_15
- Jangra S., Yuen K.S., Botelgo M.G., Jin D.Y. Epstein-Barr virus and innate immunity: Friends or foes? Microorganisms. 2019; 7(6): pii E183. DOI: http://doi.org/10.3390/microorganisms7060183
- Hussain T., Mulherkar R. Lymphoblastoid cell lines: a continuous in vitro source of cells to study carcinogen sensitivity and DNA repair. Int. J. Mol. Cell Med. 2012; 1(2): 75-87.
- Атаханов А.А., Сарымсаков А.А., Рашидова С.Ш. Наносистемы целлюлозы и серебра: синтез, структура и свойства. Ташкент; 2016.
- Наровлянский А.Н., Мезенцева М.В., Суетина И.А., Руссу Л.И., Иванова А.М., Полосков В.В. и др. Цитокин-регулирующая активность противовирусного препарата Целагрип в перевиваемых В-клеточных линиях лимфомы Беркитта. Вопросы вирусологии. 2019; 64(4): 165-72. DOI: http://doi.org/10.36233/0507-4088-2019-64-4-165-172
- Hinuma Y., Konn M., Yamaguchi J., Grace J.T. Replication of herpes-type virus in a Burkitt lymphoma cell line. J. Virol. 1967; 1(6): 1045-51.
- Klein E., Klein G., Nadkarni J.S., Nadkarni J.J., Wigzell H., Clifford P. Surface IgM kappa specificity on a Burkitt lymphoma cell in vivo and in derived cultured lines. Cancer Res. 1968; 28(7): 1300-10.
- Шувалов А.Н., Соколова Т.М., Шаповал И.М., Ершов Ф.И. Модуляция транскрипции клеточных генов препаратом иммуномакс: активация генов интерферонов и интерлейкинов. Иммунология. 2014; 35(1): 16-20.
- Соколова Т.М., Шувалов А.Н., Полосков В.В., Ершов Ф.И. Стимуляция генов сигнальной трансдукции препаратами «Ридостин», «Циклоферон» и «Ингавирин». Цитокины и воспаление. 2015; 14(2): 26-34.
- Соколова Т.М., Шувалов А.Н., Колодяжная Л.В., Оспельникова Т.П., Ершов Ф.И. Механизмы действия препарата «Кагоцел» в клетках человека. Сообщение 1. Регуляция транскрипции генов системы интерферона и апоптоза. В кн.: Ершов Ф.И., Наровлянский А.Н., ред. Интерферон – 2011. М.; 2012: 389-401.
- Соколова Т.М., Кособокова Е.Н., Шувалов А.Н., Шаповал И.М., Косоруков В.С., Ершов Ф.И. Активность генов системы интерферона в клетках аденокарциномы толстого кишечника htc116: регуляция рекомбинантными интерферонами альфа2 из бактериальных и растительных продуцентов. Российский биотерапевтический журнал. 2013; 12(3): 39-44.
- Li L.D., Sun H.F., Liu X.X., Gao S.P., Jiang H.L., Hu X., et al. Down-regulation of NDUFB9 promotes breast cancer cell proliferation, metastasis by mediating mitochondrial metabolism. PLoS One. 2015; 10(12): e0144441. DOI: http://doi.org/10.1371/journal.pone.0144441
- Измайлов Д.Ю., Владимиров Г.К. Хемилюминесценция как метод изучения свободных радикалов, глава 8. В кн.: Владимиров Ю.А., ред. Источники и мишени свободных радикалов в крови человека. М.: МАКС Пресс; 2017: 273-97.
- Toufektchan E., Toledo F. The Guardian of the genome revisited: P53 downregulates genes required for telomere maintenance, DNA repair, and centromere structure. Cancers (Basel). 2018; 10(5): pii E135. DOI: http://doi.org/10.3390/cancers10050135
- Чумаков П.М. Белок р53 и его универсальные функции в многоклеточном организме. Успехи биологической химии. 2007; 47(1): 3-52.
- Davis R.E., Brown K.D., Siebenlist U., Staudt L.M. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J. Exp. Med. 2001; 194(12): 1861-74. DOI: http://doi.org/10.1084/jem.194.12.1861
- Stephenson H.N., Herzig A., Zychlinsky A. Beyond the grave: When is cell death critical for immunity to infection? Curr. Opin. Immunol. 2016; 38: 59-66. DOI: http://doi.org/10.1016/j.coi.2015.11.004
- Jorgensen I., Rayamajhi M., Miao E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017; 17(3): 151-64. DOI: http://doi.org/10.1038/nri.2016.147
- Der S.D., Zhou A., Williams B.R., Silverman R.H. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA. 1998; 95(26): 15623-8. DOI: http://doi.org/10.1073/pnas.95.26.15623
- Chang J., Renne R., Dittmer D., Ganem D. Inflammatory cytokines and the reactivation of Kaposi’s sarcoma-associated herpesvirus lytic replication. Virology. 2000; 266(1): 17-25. DOI: http://doi.org/10.1006/viro.1999.0077
- Zhang D., Zhang D.E. Interferon-stimulated gene 15 and the protein ISGylation system. J. Interferon Cytokine Res. 2011; 31(1): 119-30. DOI: http://doi.org/10.1089/jir.2010.0110
- Dos Santos P.F., Mansur D.S. Beyond ISGlylation: Functions of free intracellular and extracellular ISG15. J. Interferon Cytokine Res. 2017; 37(6): 246-53. DOI: http://doi.org/10.1089/jir.2016.0103
- Albert M., Bécares M., Falqui M., Fernández-Lozano C., Guerra S. ISG15, a small molecule with huge implications: regulation of mitochondrial homeostasis. Viruses. 2018; 10(11): pii E629. DOI: http://doi.org/10.3390/v10110629
- Wang J., Nagy N., Masucci M.G. The Epstein-Barr virus nuclear antigen-1 upregulates the cellular antioxidant defense to enable B-cell growth transformation and immortalization. Oncogene. 2020; 39(3): 603-6. DOI: http://doi.org/10.1038/s41388-019-1003-3
- Villarroya-Beltri С., Guerra S., Sánchez-Madri F. ISGylation – a key to lock the cell gates for preventing the spread of threats. J. Cell Sci. 2017; 130(18): 2961-9. DOI: http://doi.org/10.1242/jcs.205468
- Sen G.C., Sarkar S.N. The interferon-stimulated genes: targets of direct signaling by interferons, double-stranded RNA, and viruses. Curr. Top. Microbiol. Immunol. 2007; 316: 233-50. DOI: http://doi.org/10.1007/978-3-540-71329-6_12
- Park J.H., Yang S.W., Park J.M., Ka S.H., Kim J.H., Kong Y.Y., et al. Positive feedback regulation of p53 transactivity by DNA damageinduced ISG15 modification. Nat. Commun. 2016; 7: 12513. DOI: http://doi.org/10.1038/ncomms12513
- Hummer B.T., Li X.L., Hassel B.A. Role for p53 in gene induction by double-stranded RNA. J. Virol. 2001; 75(16): 7774-7. DOI: http://doi.org/10.1128/JVI.75.16.7774-7777.2001
- Liu C., Chang R., Yao X., Qiao W.T., Geng Y.Q. ISG15 expression in response to double-stranded RNA or LPS in cultured fetal bovine lung (FBL) cells. Vet. Res. Commun. 2009; 33(7): 723-33. DOI: http://doi.org/10.1007/s11259-009-9221-8
- Chairatvit K., Wongnoppavich A., Choonate S. Up-regulation of interferon-stimulated gene15 and its conjugates by tumor necrosis factor-alpha via type I interferon-dependent and -independent pathways. Mol. Cell. Biochem. 2012; 368(1-2): 195-201. DOI: http://doi.org/10.1007/s11010-012-1360-5
- Liu F., Gao X., Wang J., Gao C., Li X., Li X., et al. Transcriptome sequencing to identify transcription factor regulatory network and alternative splicing in endothelial cells under VEGF stimulation. J. Mol. Neurosci. 2016; 58(2): 170-7. DOI: http://doi.org/10.1007/s12031-015-0653-z
- Doyle S.E., Vaidya S.A., O’Connell R., Dadgostar H., Dempsey P.W., Wu T.T., et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002; 17(3): 251-63. DOI: http://doi.org/10.1016/S1074-7613(02)00390-4
- Taylor J.L., D’Cunha J., Tom P., O’Brien W.J., Borden E.C. Production of ISG-15, an interferon-inducible protein, in human corneal cells. J. Interferon Cytokine Res. 1996; 16(11): 937-40. DOI: http://doi.org/10.1089/jir.1996.16.937
- Park J.H., Yang S.W., Park J.M., Ka S.H., Kim J.H., Kong Y.Y., et al. Positive feedback regulation of p53 transactivity by DNA damageinduced ISG15 modification. Nat. Commun. 2016; 7: 12513. DOI: http://doi.org/10.1038/ncomms12513
- Nakka V.P., Lang B.T., Lenschow D.J., Zhang D.E., Dempsey R.J., Vemuganti R. Increased cerebral protein ISGylation after focal ischemia is neuroprotective. J. Cereb. Blood Flow Metab. 2011; 31(12): 2375-84. DOI: http://doi.org/10.1038/jcbfm.2011.103
- Lou Z., Wei J., Riethman H., Baur J.A., Voglauer R., Shay J.W., et al. Telomere length regulates ISG15 expression in human cells. Aging (Albany NY). 2009; 1(7): 608-21. DOI: http://doi.org/10.18632/aging.100066
- Kiessling A., Hogrefe C., Erb S., Bobach C., Fuessel S., Wessjohann L., et al. Expression, regulation and function of the ISGylation system in prostate cancer. Oncogene. 2009; 28(28): 2606-20. DOI: http://doi.org/10.1038/onc.2009.115
- Li C., Wang J., Zhang H., Zhu M., Chen F., Hu Y., et al. Interferon-stimulated gene 15 (ISG15) is a trigger for tumorigenesis and metastasis of hepatocellular carcinoma. Oncotarget. 2014; 5(18): 8429-41. DOI: http://doi.org/10.18632/oncotarget.2316
- Wood L.M., Pan Z.K., Seavey M.M., Muthukumaran G., Paterson Y. The ubiquitin-like protein, ISG15, is a novel tumor-associated antigen for cancer immunotherapy. Cancer Immunol. Immunother. 2012; 61(5): 689-700. DOI: http://doi.org/10.1007/s00262-011-1129-9
Supplementary files