Review of candidate vaccines for the prevention of Lassa fever
- Authors: Popova O.D.1, Zubkova O.V.1, Ozharovskaia T.A.1, Zrelkin D.I.1, Voronina D.V.1, Dolzhikova I.V.1, Shcheblyakov D.V.1, Naroditsky B.S.1, Logunov D.Y.1, Gintsburg A.L.1
-
Affiliations:
- FSBI «National Research Centre for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya» of the Ministry of Health of Russia
- Issue: Vol 66, No 2 (2021)
- Pages: 91-102
- Section: REVIEWS
- URL: https://journal-vniispk.ru/0507-4088/article/view/118140
- DOI: https://doi.org/10.36233/0507-4088-33
- ID: 118140
Cite item
Full Text
Abstract
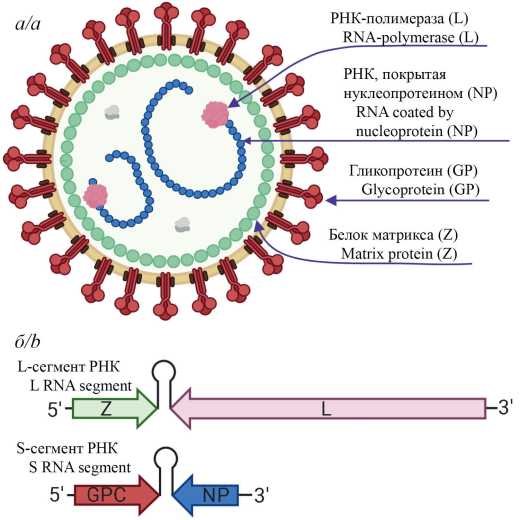
The Lassa virus one of the main etiological agent of hemorrhagic fevers in the world: according to WHO estimates, it affects 100,000 to 300,000 people annually, which results in up to 10,000 deaths [1]. Although expansion of Lassa fever caused by this pathogen is mostly limited to the West African countries: Sierra Leone, Liberia, Guinea and Nigeria, imported cases have been historically documented in Europe, the United States of America (USA), Canada, Japan, and Israel [2]. In 2017, WHO included the Lassa virus in the list of priority pathogens in need of accelerated research, development of vaccines, therapeutic agents and diagnostic tools regarding infections they cause [3]. This review describes main technological platforms used for the development of vaccines for the prevention of Lassa fever.
Full Text
##article.viewOnOriginalSite##About the authors
O. D. Popova
FSBI «National Research Centre for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya» of the Ministry of Health of Russia
Author for correspondence.
Email: olga.popova31@yandex.ru
ORCID iD: 0000-0003-3248-1227
Ol’ga D. Popova, junior researcher, Immunobiotechnology Laboratory
123098, Moscow
Russian FederationO. V. Zubkova
FSBI «National Research Centre for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya» of the Ministry of Health of Russia
Email: olga-zubkova@yandex.ru
ORCID iD: 0000-0001-7893-8419
Ol’ga V. Zubkova, PhD, senior researcher, Immunobiotechnology Laboratory
123098, Moscow
Russian FederationT. A. Ozharovskaia
FSBI «National Research Centre for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya» of the Ministry of Health of Russia
Email: o.tatiana09@yahoo.com
ORCID iD: 0000-0001-7147-1553
Tatiana A. Ozharovskaia, junior researcher, Molecular biotechnology Laboratory
123098, Moscow
Russian FederationD. I. Zrelkin
FSBI «National Research Centre for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya» of the Ministry of Health of Russia
Email: aleza4striker@gmail.com
ORCID iD: 0000-0003-0899-8357
Denis I. Zrelkin, junior researcher, Immunobiotechnology Laboratory
123098, Moscow
Russian FederationD. V. Voronina
FSBI «National Research Centre for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya» of the Ministry of Health of Russia
Email: daryavoronin2009@yandex.ru
ORCID iD: 0000-0001-6629-744X
Daria V. Voronina, junior researcher, Immunobiotechnology Laboratory
123098, Moscow
Russian FederationI. V. Dolzhikova
FSBI «National Research Centre for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya» of the Ministry of Health of Russia
Email: i.dolzhikova@gmail.com
ORCID iD: 0000-0003-2548-6142
Inna V. Dolzhikova, PhD, Head of the Laboratory of the State Collection of Viruses
123098, Moscow
Russian FederationD. V. Shcheblyakov
FSBI «National Research Centre for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya» of the Ministry of Health of Russia
Email: sdmitryv@yahoo.com
ORCID iD: 0000-0002-1289-3411
Dmitry V. Shcheblyakov, PhD, lead researcher
123098, Moscow
Russian FederationB. S. Naroditsky
FSBI «National Research Centre for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya» of the Ministry of Health of Russia
Email: bsnar1941@yahoo.com
ORCID iD: 0000-0001-5522-8238
Boris S. Naroditsky, DSc, professor, main researcher
123098, Moscow
Russian FederationD. Yu. Logunov
FSBI «National Research Centre for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya» of the Ministry of Health of Russia
Email: ldenisy@yahoo.com
ORCID iD: 0000-0003-4035-6581
Denis Yu. Logunov, DSc, Corresponding Member of the Russian Academy of Sciences, Deputy Director for Science
123098, Moscow
Russian FederationA. L. Gintsburg
FSBI «National Research Centre for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya» of the Ministry of Health of Russia
Email: gintsburg@gamaleya.org
ORCID iD: 0000-0003-1769-5059
Aleksander L. Gintsburg, DSc, RAS academician, professor, Director
123098, Moscow
Russian FederationReferences
- WHO. Introduction to Lassa fever. Available at: https://www.who.int/publications/i/item/introduction-to-lassa-fever (accessed December 15, 2020).
- Wolf T., Ellwanger R., Goetsch U., Wetzstein N., Gottschalk R. Fifty years of imported Lassa fever: a systematic review of primary and secondary cases. J. Travel Med. 2020; 27(4): 1–17. https://doi.org/10.1093/jtm/taaa035.
- WHO. WHO Target Product Profile for Lassa virus Vaccine; June 2017. Available at: https://www.who.int/blueprint/priority-diseases/key-action/LassaVirusVaccineTPP.PDF (accessed December 15, 2020).
- Roberts L. Nigeria hit by unprecedented Lassa fever outbreak. Science. 2018; 359(6381): 1201–2. https://doi.org/10.1126/science.359.6381.1201.
- Coulibaly-N’Golo D., Allali B., Kouassi S.K., Fichet-Calvet E., Becker-Ziaja B, Rieger T., et al. Novel arenavirus sequences in Hylomyscus sp. and Mus (Nannomys) setulosus from Côte d’Ivoire: Implications for evolution of arenaviruses in Africa. PLoS One. 2011; 6(6): e20893. https://doi.org/10.1371/journal.pone.0020893.
- McCormick J.B., Webb P.A., Krebs J.W., Johnson K.M., Smith E.S. A prospective study of the epidemiology and ecology of Lassa fever carried out primarily in the eastern province of Sierra. J. Infect. Dis. 1987; 155(3): 437–44. https://doi.org/10.1093/infdis/155.3.437.
- Okokhere P., Colubri A., Azubike C., Iruolagbe C., Osazuwa O., Tabrizi S., et al. Clinical and laboratory predictors of Lassa fever outcome in a dedicated treatment facility in Nigeria: an observational cohort study. Lancet Infect. Dis. 2019; 18(6): 684–95. https://doi.org/10.1016/s1473-3099(18)30121-x.
- Asogun D.A., Günther S., Akpede G.O., Ihekweazu C., Zumla A. Lassa fever: epidemiology, clinical features, diagnosis, management and prevention. Infect. Dis. Clin. North Am. 2019; 33(4): 933–51. https://doi.org/10.1016/j.idc.2019.08.002.
- Mateer E.J., Huang C., Shehu N.Y., Paessler S. Lassa fever-induced sensorineural hearing loss: A neglected public health and social burden. PLoS Negl. Trop. Dis. 2018; 12(2): e0006187. https://doi.org/10.1371/journal.pntd.0006187.
- Okogbenin S., Okoeguale J., Akpede G., Colubri A., Barnes K.G., Mehta S., et al. Retrospective cohort study of Lassa fever in pregnancy, Southern Nigeria. Emerg. Infect. Dis. 2019; 25(8): 1495–500. https://doi.org/10.3201/eid2508.181299.
- Buba M.I., Dalhat M.M., Nguku P.M., Waziri N., Mohammad J.O., Bomoi I.M., et al. Mortality among confirmed Lassa fever cases during the 2015–2016 outbreak in Nigeria. Am. J. Public Health. 2018; 108(2): 262–4. https://doi.org/10.2105/ajph.2017.304186.
- NCDC. Lassa fever Situation Report. 2020. Available at: https://ncdc.gov.ng/themes/common/files/sitreps/15a12399a0aa98330e56dabd49ccefb8.pdf (accessed December 15, 2020).
- Radoshitzky S.R., Buchmeier M.J., Charrel R.N., Clegg J.C.S., Gonzalez J.J., Günther S., et al. ICTV virus taxonomy profile: Arenaviridae. J. Gen. Virol. 2019; 100(8): 1200–1. https://doi.org/10.1099/jgv.0.001280.
- Klitting R., Mehta S.B., Oguzie J.U., Oluniyi P.E., Pauthner M.G., Siddle K.J., et al. Lassa Virus Genetics. Curr. Top. Microbiol. Immunol. 2020, online ahead of print. https://doi.org/10.1007/82_2020_212.
- Zinzula L., Tramontano E. Strategies of highly pathogenic RNA viruses to block dsRNA detection by RIG-I-like receptors: Hide, mask, hit. Antiviral Res. 2013; 100(3): 615–35. https://doi.org/10.1016/j.antiviral.2013.10.002.
- Lenz O., ter Meulen J., Klenk H.D., Seidah N.G., Garten W. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc. Natl. Acad. Sci. USA. 2001; 98(22): 12701–5. https://doi.org/10.1073/pnas.221447598.
- Knipe D.M., Howley P. Fields Virology. New York: Lippincott Williams & Wilkins; 2013.
- Mateo M., Reynard S., Carnec X., Journeaux A., Baillet N., Schaeffer J., et al. Vaccines inducing immunity to Lassa virus glycoprotein and nucleoprotein protect macaques after a single shot. Sci. Transl. Med. 2019; 11(512): eaaw3163. https://doi.org/10.1126/scitranslmed.aaw3163.
- Whitmer S.L.M., Strecker T., Cadar D., Dienes H.P., Faber K., Patel K., et al. New lineage of Lassa virus, Togo, 2016. Emerg. Infect. Dis. 2018; 24(3): 599–602. https://doi.org/10.3201/eid2403.171905.
- McLay L., Liang Y., Ly H. Comparative analysis of disease pathogenesis and molecular mechanisms of New World and Old World arenavirus infections. J. Gen. Virol. 2014; 95(Pt. 1): 1–15. https://doi.org/10.1099/vir.0.057000-0.
- Baize S., Kaplon J., Faure C., Pannetier D., Georges-Courbot M.C., Deubel V. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J. Immunol. 2004; 172(5): 2861–9. https://doi.org/10.4049/jimmunol.172.5.2861.
- Hastie K.M., Kimberlin C.R., Zandonatti M.A., MacRae I.J., Saphire E.O. Structure of the Lassa virus nucleoprotein reveals a dsRNA-specific 3’ to 5’ exonuclease activity essential for immune suppression. Proc. Natl. Acad. Sci. USA. 2011; 108(6): 2396–401. https://doi.org/10.1073/pnas.1016404108.
- Pythoud C., Rodrigo W.W., Pasqual G., Rothenberger S., MartínezSobrido L., de la Torre J.C., et al. Arenavirus nucleoprotein targets interferon regulatory factor-activating kinase IKKε. J. Virol. 2012; 86(15): 7728–38. https://doi.org/10.1128/jvi.00187-12.
- Xing J., Ly H., Liang Y. The Z proteins of pathogenic but not nonpathogenic arenaviruses inhibit RIG-I-like receptor-dependent interferon production. J. Virol. 2015; 89(5): 2944–55. https://doi.org/10.1128/jvi.03349-14.
- Zapata J.C., Medina-Moreno S., Guzmán-Cardozo C., Salvato M.S. Improving the breadth of the host’s immune response to Lassa virus. Pathogens. 2018; 7(4): 84. https://doi.org/10.3390/pathogens7040084.
- Sommerstein R., Flatz L., Remy M.M., Malinge P., Magistrelli G., Fischer N., et al. Arenavirus glycan shield promotes neutralizing antibody evasion and protracted infection. PLoS Pathog. 2015; 11(11): e1005276. https://doi.org/10.1371/journal.ppat.1005276.
- Russier M., Pannetier D., Baize S. Immune responses and Lassa virus infection. Viruses. 2012; 4(11): 2766–85. https://doi.org/10.3390/v4112766.
- Prescott J.B., Marzi A., Safronetz D., Robertson S.J., Feldmann H., Best S.M. Immunobiology of Ebola and Lassa virus infections. Nat. Rev. Immunol. 2017; 17(3): 195–207. https://doi.org/10.1038/nri.2016.138.
- Salami K., Gouglas D., Schmaljohn C., Saville M., Tornieporth N.A review of Lassa fever vaccine candidates. Curr. Opin. Virol. 2019; 37: 105–11. https://doi.org/10.1016/j.coviro.2019.07.006.
- Search of_ Lassa – List Results – ClinicalTrials.gov. Available at: https://www.clinicaltrials.gov/ct2/results?term=Lassa&Search (accessed December 15, 2020).
- Stein D.R., Warner B.M., Soule G., Tierney K., Frost K.L., Booth S. A recombinant vesicular stomatitis-based Lassa fever vaccine elicits rapid and long-term protection from lethal Lassa virus infection in guinea pigs. NPJ Vaccines. 2019; 4: 8. https://doi.org/10.1038/s41541-019-0104-x.
- Marzi A., Feldmann F., Geisbert TW., Feldmann H., Safronetz D. Vesicular stomatitis virus-based vaccines against Lassa and Ebola viruses. Emerg. Infect. Dis. 2015; 21(2): 305–7. https://doi.org/10.3201/eid2102.141649.
- Geisbert T.W., Jones S., Fritz E.A., Shurtleff A.C., Geisbert J.B., Liebscher R., et al. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2005; 2(6): 0537–45. https://doi.org/10.1371/journal.pmed.0020183.
- Safronetz D., Mire C., Rosenke K., Feldmann F., Haddock E., Geisbert T., et al. A recombinant vesicular stomatitis virus-based Lassa fever vaccine protects guinea pigs and macaques against challenge with geographically and genetically distinct Lassa viruses. PLoS Negl. Trop. Dis. 2015; 9(4): e0003736. https://doi.org/10.1371/journal.pntd.0003736.
- Cross R.W., Xu R., Matassov D., Hamm S., Latham T.E., Gerardi C.S., et al. Quadrivalent VesiculoVax vaccine protects nonhuman primates from viral-induced hemorrhagic fever and death. J. Clin. Invest. 2020; 130(1): 539–51. https://doi.org/10.1172/jci131958.
- Clarke D.K., Xu R., Matassov D., Latham T.E., Ota-Setlik A., Gerardi C.S., et al. Safety and immunogenicity of a highly attenuated rVSVN4CT1-EBOVGP1 Ebola virus vaccine: a randomised, double-blind, placebo-controlled, phase 1 clinical trial. Lancet Infect. Dis. 2020; 20(4): 455–66. https://doi.org/10.1016/s1473-3099(19)30614-0.
- Clegg J.C.S., Lloyd G. Vaccinia recombinant expressing Lassa-virus internal nucleocapsid protein protects guineapigs against Lassa fever. Lancet. 1987; 330(8552): 186–8. https://doi.org/10.1016/s0140-6736(87)90767-7.
- Morrison H.G., Bauer S.P., Lange J.V., Esposito J.J., McCormick J.B., Auperin D.D., et al. Protection of guinea pigs from Lassa fever by vaccinia virus recombinants expressing the nucleoprotein or the envelope glycoproteins of Lassa virus. Virology. 1989; 171(1): 179–88. https://doi.org/10.1016/0042-6822(89)90525-4.
- Morrison H.G., Goldsmith C.S., Regnery H.L., Auperin D.D. Simultaneous expression of the Lassa virus N and GPC genes from a single recombinant vaccinia virus. Virus Res. 1991; 18(2-3): 231–41. https://doi.org/10.1016/0168-1702(91)90021-m.
- Cottingham M.G., Carroll M.W. Recombinant MVA vaccines: Dispelling the myths. Vaccine. 2013; 31(39): 4247–51. https://doi.org/10.1016/j.vaccine.2013.03.021.
- Salvato M.S., Domi A., Guzmán-Cardozo C., Medina-Moreno S., Zapata J.C., Hsu H., et al. A single dose of modified vaccinia Ankara expressing Lassa virus-like particles protects mice from lethal intracerebral virus challenge. Pathogens. 2019; 8(3): 133. https://doi.org/10.3390/pathogens8030133.
- Giel-Moloney M., Goncalvez A.P., Catalan J., Lecouturier V., GirerdChambaz Y., Diaz F., et al. Chimeric yellow fever 17D-Zika virus (ChimeriVax-Zika) as a live-attenuated Zika virus vaccine. Sci. Rep. 2018; 8(1): 13206. https://doi.org/10.1038/s41598-018-31375-9.
- Bonaldo M.C., Sequeira P.C., Galler R. The yellow fever 17D virus as a platform for new live attenuated vaccines. Hum. Vaccines Immunother. 2014; 10(5): 1256–65. https://doi.org/10.4161/hv.28117.
- Bredenbeek P.J., Molenkamp R., Spaan W.J., Deubel V., Marianneau P., Salvato M.S., et al. A recombinant Yellow Fever 17D vaccine expressing Lassa virus glycoproteins. Virology. 2006; 345(2): 299–304. https://doi.org/10.1016/j.virol.2005.12.001.
- Jiang X., Dalebout T.J., Bredenbeek P.J., Carrion R. Jr., Brasky K., Patterson J., et al. Yellow fever 17D-vectored vaccines expressing Lassa virus GP1 and GP2 glycoproteins provide protection against fatal disease in guinea pigs. Vaccine. 2011; 29(6): 1248–57. https://doi.org/10.1016/j.vaccine.2010.11.079.
- Busch E., Kubon K.D., Mayer J.K.M., Pidelaserra-Martí G., Albert J., Hoyler B., et al. Measles vaccines designed for enhanced CD8+ T cell activation. Viruses. 2020; 12(2): 242. https://doi.org/10.3390/v12020242.
- A Trial to Evaluate the Optimal Dose of MV-LASV. Available at: https://clinicaltrials.gov/ct2/show/NCT04055454 (accessed December 15, 2020).
- Crystal R.G. Adenovirus: The first effective in vivo gene delivery vector. Hum. Gene Ther. 2014; 25(1): 3–11. https://doi.org/10.1089/hum.2013.2527.
- Maruyama J., Mateer E.J., Manning J.T., Sattler R., Seregin A.V., Bukreyeva N., et al. Adenoviral vector-based vaccine is fully protective against lethal Lassa fever challenge in Hartley guinea pigs. Vaccine. 2019; 37(45): 6824–31. https://doi.org/10.1016/j.vaccine.2019.09.030.
- Lukashevich I.S., Patterson J., Carrion R., Moshkoff D., Ticer A., Zapata J., et al. A live attenuated vaccine for Lassa fever made by reassortment of Lassa and Mopeia viruses. J. Virol. 2005; 79(22): 13934–42. https://doi.org/10.1128/jvi.79.22.13934-13942.2005.
- Carrion R. Jr., Patterson J.L., Johnson C., Gonzales M., Moreira C.R., Ticer A., et al. A ML29 reassortant virus protects guinea pigs against a distantly related Nigerian strain of Lassa virus and can provide sterilizing immunity. Vaccine. 2007; 25(20): 4093–102. https://doi.org/10.1016/j.vaccine.2007.02.038.
- Zapata J.C., Poonia B., Bryant J., Davis H., Ateh E., George L., et al. An attenuated Lassa vaccine in SIV-infected rhesus macaques does not persist or cause arenavirus disease but does elicit Lassa virus-specific immunity. Virol. J. 2013; 10: 52. https://doi.org/10.1186/1743-422x-10-52.
- Zhang L., Marriott K.A., Harnish D.G., Aronson J.F. Reassortant analysis of guinea pig virulence of Pichinde virus variants. Virology. 2001; 290(1): 30–8. https://doi.org/10.1006/viro.2001.1127.
- Lukashevich I.S. Generation of reassortants between African arenaviruses. Virology. 1992; 188(2): 600–5. https://doi.org/10.1016/0042-6822(92)90514-p.
- Riviere Y., Ahmed R., Southern P.J., Buchmeier M.J., Oldstone M.B. Genetic mapping of lymphocytic choriomeningitis virus pathogenicity: virulence in guinea pigs is associated with the L RNA segment. J. Virol. 1985; 55(3): 704–9. https://doi.org/10.1128/jvi.55.3.704-709.1985.
- Gary E.N., Weiner D.B. DNA vaccines: prime time is now. Curr. Opin. Immunol. 2020; 65: 21–7. https://doi.org/10.1016/j.coi.2020.01.006.
- Smith T.R.F., Patel A., Ramos S., Elwood D., Zhu X., Yan J., et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020; 11(1): 2601. https://doi.org/10.1038/s41467-020-16505-0.
- Cashman K., Broderick K.E., Wilkinson E.R., Shaia C.I., Bell T.M., Shurtleff A.C., et al. Enhanced efficacy of a codon-optimized DNA vaccine encoding the glycoprotein precursor gene of Lassa virus in a Guinea pig disease model when delivered by dermal electroporation. Vaccines (Basel). 2013; 1(3): 262–77. https://doi.org/10.3390/vaccines1030262.
- Cashman K.A., Wilkinson E.R., Shaia C.I., Facemire P.R., Bell T.M., Bearss J.J., et al. A DNA vaccine delivered by dermal electroporation fully protects cynomolgus macaques against Lassa fever. Hum. Vaccines Immunother. 2017; 13(12): 2902–11. https://doi.org/10.1080/21645515.2017.1356500.
- Safety, Tolerability and Immunogenicity of INO-4800 in Healthy Volunteers. Available at: https://clinicaltrials.gov/ct2/show/NCT04336410 (accessed December 15, 2020).
- Dose-ranging Study: Safety, Tolerability and Immunogenicity of INO-4500 in Healthy Volunteers in Ghana; 2019. Available at: https://clinicaltrials.gov/ct2/show/NCT04093076 (accessed December 15, 2020).
- McCormick J.B., Mitchell S.W., Kiley M.P., Ruo S., Fisher-Hoch S.P. Inactivated Lassa virus elicits a non protective immune response in rhesus monkeys. J. Med. Virol. 1992; 37(1): 1–7. https://doi.org/10.1002/jmv.1890370102.
- Cai Y., Iwasaki M., Motooka D., Liu D.X., Yu S., Cooper K., et al. A lassa virus live-attenuated vaccine candidate based on rearrangement of the intergenic region. mBio. 2020; 11(2): e00186–20. https://doi.org/10.1128/mbio.00186-20.
- Cai Y., Ye C., Cheng B., Nogales A., Iwasaki M., Yu S., et al. A lassa fever live-attenuated vaccine based on codon deoptimization of the viral glycoprotein gene. mBio. 2020; 11(1): e00039–20. https://doi.org/10.1128/mbio.00039-20.
- Kainulainen M.H., Spengler J.R., Welch S.R., Coleman-McCray J.D., Harmon J.R., Klena J.D., et al. Use of a scalable replicon-particle vaccine to protect against lethal Lassa virus infection in the Guinea pig model. J. Infect. Dis. 2019; 217(12): 1957–66. https://doi.org/10.1093/infdis/jiy123.
- Pushko P., Geisbert J., Parker M., Jahrling P., Smith J. Individual and bivalent vaccines based on alphavirus replicons protect Guinea pigs against infection with Lassa and Ebola viruses. J. Virol. 2001; 75(23): 11677–85. https://doi.org/10.1128/jvi.75.23.11677-11685.2001.
Supplementary files