Specific features of the pathology of the respiratory system in SARS-CoV-2 (Coronaviridae: Coronavirinae: Betacoronavirus: Sarbecovirus) infected Syrian hamsters (Mesocricetus auratus)
- Authors: Chepur S.V.1, Alekseeva I.I.1, Vladimirova O.O.1, Myasnikov V.A.1, Tyunin M.A.1, Ilinskii N.S.1, Nikishin A.S.1, Shevchenko V.A.1, Smirnova A.V.1
-
Affiliations:
- FSBI «State Research Testing Institute of Military Medicine» of the Ministry of Defense of the Russian Federation
- Issue: Vol 66, No 6 (2021)
- Pages: 442-451
- Section: ORIGINAL RESEARCHES
- URL: https://journal-vniispk.ru/0507-4088/article/view/118216
- DOI: https://doi.org/10.36233/0507-4088-63
- ID: 118216
Cite item
Full Text
Abstract
Introduction. Verification of histological changes in respiratory system using Syrian (golden) hamsters (Mesocricetus auratus) as experimental model is an important task for preclinical studies of drugs intended for prevention and treatment of the novel coronavirus infection COVID-19.
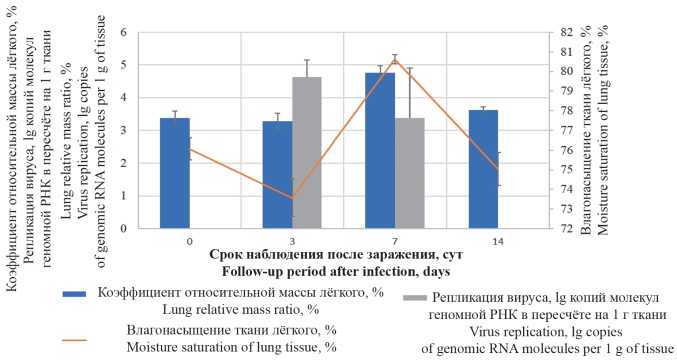
The aim of this work was to study pathological changes of pulmonary tissue in SARS-CoV-2 (Coronaviridae: Coronavirinae: Betacoronavirus; Sarbecovirus) experimental infection in Syrian hamsters. Material and methods. Male Syrian hamsters weighting 80–100 g were infected by intranasal administration of culture SARS-CoV-2 at dose 4 × 104 TCID50/ml (TCID is tissue culture infectious dose). Animals were euthanatized on 3, 7 and 14 days after infection, with gravimetric registration. The viral load in lungs was measured using the polymerase chain reaction (PCR). Right lung and trachea tissues were stained with hematoxylin-eosin and according to Mallory.
Results and discussion. The highest viral replicative activity in lungs was determined 3 days after the infection. After 7 days, on a background of the decrease of the viral load in lungs, a pathologically significant increase of the organ’s gravimetric parameters was observed. Within 3 to 14 days post-infection, the lung histologic pattern had been showing the development of inflammation with a succession of infiltrative-proliferative, edematousmacrophagal and fibroblastic changes. It was found that initial changes in respiratory epithelium can proceed without paranecrotic interstitial inflammation, while in the formation of multiple lung parenchyma lesions, damage to the epithelium of bronchioles and acinar ducts can be secondary. The appearance of epithelioid large-cell metaplastic epithelium, forming pseudoacinar structures, was noted as a pathomorphological feature specific to SARS-CoV-2 infection in Syrian hamsters.
Conclusion. As a result of the study, the specific features of the pathology of the respiratory system in SARSCoV-2 infected Syrian hamsters were described. These findings are of practical importance as reference data that can be used for preclinical studies to assess the effectiveness of vaccines and potential drugs.
Full Text
##article.viewOnOriginalSite##About the authors
S. V. Chepur
FSBI «State Research Testing Institute of Military Medicine» of the Ministry of Defense of the Russian Federation
ORCID iD: 0000-0002-5324-512X
195043, Saint Petersburg, Russia
Russian FederationI. I. Alekseeva
FSBI «State Research Testing Institute of Military Medicine» of the Ministry of Defense of the Russian Federation
ORCID iD: 0000-0002-0924-9158
195043, Saint Petersburg, Russia
Russian FederationO. O. Vladimirova
FSBI «State Research Testing Institute of Military Medicine» of the Ministry of Defense of the Russian Federation
ORCID iD: 0000-0002-8703-6799
195043, Saint Petersburg, Russia
Russian FederationV. A. Myasnikov
FSBI «State Research Testing Institute of Military Medicine» of the Ministry of Defense of the Russian Federation
ORCID iD: 0000-0001-7232-4678
195043, Saint Petersburg, Russia
Russian FederationM. A. Tyunin
FSBI «State Research Testing Institute of Military Medicine» of the Ministry of Defense of the Russian Federation
ORCID iD: 0000-0002-6974-5583
195043, Saint Petersburg, Russia
Russian FederationN. S. Ilinskii
FSBI «State Research Testing Institute of Military Medicine» of the Ministry of Defense of the Russian Federation
ORCID iD: 0000-0001-7406-753X
195043, Saint Petersburg, Russia
Russian FederationA. S. Nikishin
FSBI «State Research Testing Institute of Military Medicine» of the Ministry of Defense of the Russian Federation
Author for correspondence.
Email: nikishin1664@gmail.com
ORCID iD: 0000-0003-1372-369X
Aleksandr S. Nikishin, Researcher
195043, Saint Petersburg, Russia
Russian FederationV. A. Shevchenko
FSBI «State Research Testing Institute of Military Medicine» of the Ministry of Defense of the Russian Federation
ORCID iD: 0000-0002-6984-2914
195043, Saint Petersburg, Russia
Russian FederationA. V. Smirnova
FSBI «State Research Testing Institute of Military Medicine» of the Ministry of Defense of the Russian Federation
ORCID iD: 0000-0003-0483-5032
195043, Saint Petersburg, Russia
Russian FederationReferences
- Чепур С.В., Плужников Н.Н., Чубарь О.В., Бакулина Л.С., Литвиненко И.В., Макаров В.А., и др. Респираторные РНК-вирусы: как подготовиться к встрече с новыми пандемическими штаммами. Успехи соврем. биологии. 2020; 140(4): 359–77. https://doi.org/10.31857/S0042132420040043
- Bradley B.T., Maioli H., Johnston R., Chaudhry I., Fink S.L., Xu P.H., et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020; 396(10247): 320–32. https://doi.org/10.1016/S0140-6736(20)31305-2
- Чучалин А.Г. COVID-19 пневмония: лекция для студентов ФГАОУ ВО «Российский национальный исследовательский медицинский университет им. Н.И. Пирогова»; 2020. Available at: https://youtu.be/hTVZSwa7X5c (accessed October 14, 2021).
- Becker R.C. COVID-19-associated vasculitis and vasculopathy. J. Thromb. Thrombolysis. 2020; 50(3): 499–511. https://doi.org/10.1007/s11239-020-02230-4
- Calabrese F., Pezzuto F., Fortarezza F., Hofman P., Kern I., Panizo A., et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020; 477(3): 359–72. https://doi.org/10.1007/s00428-020-02886-6
- Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020; 15(5):700–4. https://doi.org/10.1016/j.jtho.2020.02.010
- Li M., Lei P., Zeng B., Li Z., Yu P., Fan B., et al. Coronavirus Disease (COVID-19): Spectrum of CT findings and temporal progression of the disease. Acad. Radiol. 2020; 27(5): 603–8. https://doi.org/10.1016/j.acra.2020.03.003
- Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N., et al. Chest CT findings in Coronavirus Disease-19 (COVID-19):Relationship to duration of infection. Radiology. 2020; 295(3):685–91. https://doi.org/10.1148/radiol.2020200463
- Pan F., Ye T., Sun P., Gui S., Liang B., Li L., et al. Time course of lung changes at chest CT during recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020; 295(3): 715–21. https://doi.org/10.1148/radiol.2020200370
- Liu H., Liu F., Li J., Zhang T., Wanga D., Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: Focus on pregnant women and children. J. Infect. 2020; 80(5): e7–13. https://doi.org/10.1016/j.jinf.2020.03.007
- Meng H., Xiong R., He R., Weichen L., Bo H., Lin Z., et al. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J. Infect. 2020; 81(1):e33–9. https://doi.org/10.1016/j.jinf.2020.04.004
- Mohanty S.K., Satapathy A., Naidu M.M., Mukhopadhyay S., Sharma S., Barton L.M., et al. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19) – anatomic pathology perspective on current knowledge. Diagn. Pathol. 2020; 15(1): 103. https://doi.org/10.1186/s13000-020-01017-8
- Iba T., Connors J.M., Levy J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020; 69(12): 1181–9. https://doi.org/10.1007/s00011-020-01401-6
- Zhang H., Zhou P., Wei Y., Yue H., Wang Y., Hu M., et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020; 172(9): 629–32. https://doi.org/10.7326/M20-0533
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020; 395(10234): 1417–8. https://doi.org/10.1016/S0140-6736(20)30937-5
- Jain A. COVID-19 and lung pathology. Indian J. Pathol. Microbiol. 2020; 63(2): 171–2. https://doi.org/10.4103/IJPM.IJPM_280_20
- Rizzo P., Vieceli Dalla Sega F., Fortini F., Marracino L., Rapezzi C., Ferrari R., et al. COVID-19 in the heart and the lungs: could we «Notch» the inflammatory storm? Basic Res. Cardiol. 2020; 115(3):31. https://doi.org/10.1007/s00395-020-0791-5
- Nicolai L., Leunig A., Brambs S., Kaiser R., Weinberger T., Weigand M., et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020; 142(12): 1176–89. https://doi.org/10.1161/CIRCULATIONAHA.120.048488
- Sun R., Liu H., Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J. Radiol. 2020; 21(5): 541–4. https://doi.org/10.3348/kjr.2020.0180
- Rosenke K., Meade-White K., Letko M., Clancy C., Hansen F., Liu Y., et al. Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection. Emerg. Microbes Infect. 2020; 9(1): 2673–84. https://doi.org/10.1080/22221751.2020.1858177
- Imai M., Iwatsuki-Horimoto K., Hatta M., Loeber S., Halfmann P.J., et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA. 2020; 117(28): 16587–95. https://doi.org/10.1073/pnas.2009799117
Supplementary files