Resistance to antiviral drugs in human viruses from the subfamily Betaherpesvirinae
- Authors: Demin M.V.1, Tikhomirov D.S.1, Tupoleva T.A.1, Filatov F.P.2,3
-
Affiliations:
- National Medical Research Center of Hematology of the Ministry of Health of Russia
- I.I. Mechnikov Research Institute of Vaccines and Serums of the Ministry of Education and Science of Russia
- National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya of the Ministry of Health of Russia
- Issue: Vol 67, No 5 (2022)
- Pages: 385-394
- Section: REVIEWS
- URL: https://journal-vniispk.ru/0507-4088/article/view/118233
- DOI: https://doi.org/10.36233/0507-4088-136
- ID: 118233
Cite item
Full Text
Abstract
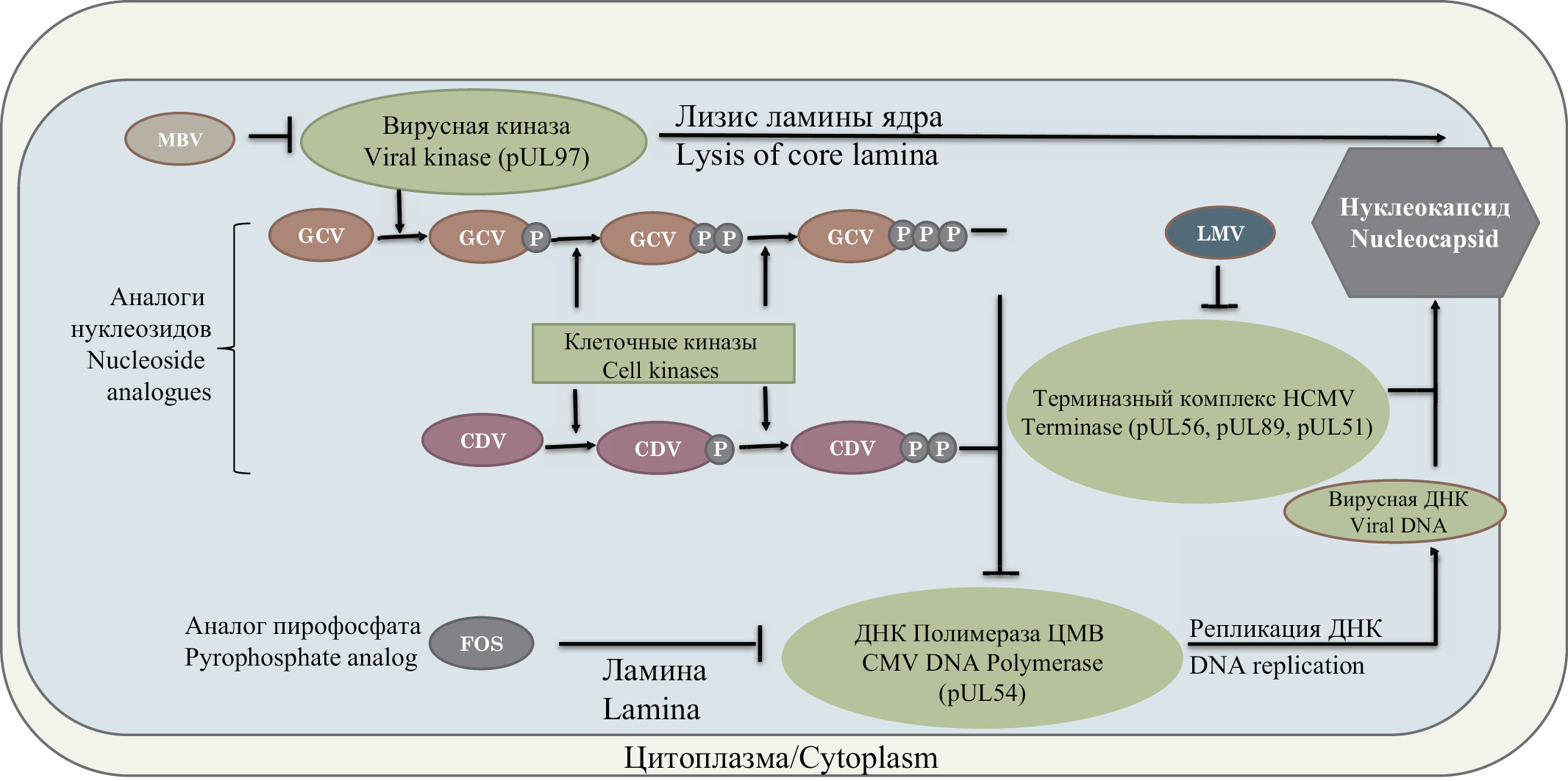
The review provides information on the mechanisms of the emergence of resistance to antiviral drugs in human viruses from the subfamily Betaherpesvirinae. Data on the principles of action of antiviral drugs and their characteristics are given. The occurrence rates of viral resistance in various groups of patients is described and information about the possible consequences of the emergence of resistance to antiviral drugs is given. Information is provided regarding the virus genes in which mutations occur that lead to viral resistance, and a list of such mutations that have described so far is given. The significance of the study of mutations leading to the resistance of the virus to antiviral drugs for medical practice is discussed.
Full Text
##article.viewOnOriginalSite##About the authors
Mikhail V. Demin
National Medical Research Center of Hematology of the Ministry of Health of Russia
Author for correspondence.
Email: memindisha@gmail.com
ORCID iD: 0000-0002-7579-3442
biologist
Russian Federation, 125167, MoscowDmitry S. Tikhomirov
National Medical Research Center of Hematology of the Ministry of Health of Russia
Email: tihomirovgnc@bk.ru
ORCID iD: 0000-0002-2553-6579
Candidate of biology, Head of the Laboratory of Virology
Russian Federation, 125167, MoscowTatiana A. Tupoleva
National Medical Research Center of Hematology of the Ministry of Health of Russia
Email: ttupoleva@mail.ru
ORCID iD: 0000-0003-4668-9379
Doctor of medicine, Head of the Virology Department
Russian Federation, 125167, MoscowFelix P. Filatov
I.I. Mechnikov Research Institute of Vaccines and Serums of the Ministry of Education and Science of Russia; National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya of the Ministry of Health of Russia
Email: ffelix001@gmail.com
ORCID iD: 0000-0002-2385-9251
Doctor of biology
Russian Federation, 105064, Moscow; 123098, MoscowReferences
- Umene K. Herpesviruses. Fukuoka Igaku Zasshi. 2001; 92(11): 361–4. (in Japanese)
- Piret J., Boivin G. Antiviral drug resistance in herpesviruses other than cytomegalovirus. Rev. Med. Virol. 2014; 24(3):186–218. https://doi.org/10.1002/rmv.1787
- Ramanan P., Razonable R.R. Cytomegalovirus infections in solid organ transplantation: A review. Infect. Chemother. 2013; 45(3): 260–71. https://doi.org/10.3947/ic.2013.45.3.260
- Pankratova O.S., Chukhlovin A.B., Zubarovskaya L.S., Afanas’ev B.V. Frequency of herpesvirus detection and risk of typical complications in allogeneic complications in allogeneic transplantation of hematopoietic stem cells. Uchenye zapiski SPbGMU im. akad. I.P. Pavlova. 2010; 17(1): 56–60. (in Russian)
- Kotton C.N. Management of cytomegalovirus infection in solid organ transplantation. Nat. Rev. Nephrol. 2010; 6(12): 711–21. https://doi.org/10.1038/nrneph.2010.141
- Takenaka K., Nishida T., Asano-Mori Y., Oshima K., Ohashi K., Mori T., et al. Cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation is associated with a reduced risk of relapse in patients with acute myeloid leukemia who survived to day 100 after transplantation: The Japan society for hematopoietic C. Biol. Blood Marrow Transplant. 2015; 21(11): 2008–16. https://doi.org/10.1016/j.bbmt.2015.07.019
- Rossi C., Delforge M.L., Jacobs F., Wissing M., Pradier O., Remmelink M., et al. Fatal primary infection due to human herpesvirus 6 variant A in a renal transplant recipient. Transplantation. 2001; 71(2): 288–92. https://doi.org/10.1097/00007890-200101270-00021
- Kidd M., Clark D., Sabin C., Andrew D., Hassan-Walker A., Sweny P., et al. Association of human herpesvirus 7 and cytomegalovirus co-infection with cytomegalovirus disease and increased rejection. Transplantation. 2000; 69(11): 2400–4. https://doi.org/10.1097/00007890-200006150-00032
- Britt W. Manifestations of human cytomegalovirus infection: Proposed mechanisms of acute and chronic disease. Curr. Top. Microbiol. Immunol. 2008; 325: 417–70. https://doi.org/10.1007/978-3-540-77349-8_23
- Cameron C.E., Raney K.D., Götte M. Viral Genome Replication. Boston: Springer; 2009.
- Chen S.J., Wang S.C., Chen Y.C. Antiviral agents as therapeutic strategies against cytomegalovirus infections. Viruses. 2019; 12(1): 21. https://doi.org/10.3390/v12010021
- Orlova S.V., Stoma I.O., Shmeleva N.P., Sivets N.V. The current state of the problem of infections caused by herpes viruses 6, 7 with different clinical forms and the possibilities of their treatment. Infektsionnye bolezni: novosti, mneniya, obuchenie. 2021; 10(2): 78–86. https://doi.org/10.33029/2305-3496-2021-10-1-78-86 (in Russian)
- Ward K.N., Hill J.A., Hubacek P., De La Camara R., Crocchiolo R., Einsele H., et al. Guidelines from the 2017 European Conference on Infections in Leukaemia for management of HHV-6 infection in patients with hematologic malignancies and after hematopoietic stem cell transplantation. Haematologica. 2019; 104(11): 2155–63. https://doi.org/10.3324/haematol.2019.223073
- Krishna B.A., Wills M.R., Sinclair J.H. Advances in the treatment of cytomegalovirus. Br. Med. Bull. 2019; 131(1): 5–17. https://doi.org/10.1093/bmb/ldz031
- Manichanh C., Olivier-Aubron C., Lagarde J.P., Aubin J.T., Bossi P., Gautheret-Dejean A., et al. Selection of the same mutation in the U69 protein kinase gene of human herpesvirus-6 after prolonged exposure to ganciclovir in vitro and in vivo. J. Gen. Virol. 2001; 82(Pt. 11): 2767–76. https://doi.org/10.1099/0022-1317-82-11-2767
- Ward K.N., Clark D.A. Roseoloviruses: human herpesviruses 6A, 6B and 7. In: Principles and Practice of Clinical Virology. Chichester: Wiley-Blackwell; 2009: 223–44.
- Goldner T., Hewlett G., Ettischer N., Ruebsamen-Schaeff H., Zimmermann H., Lischka P. The novel anticytomegalovirus compound AIC246 (letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase. J. Virol. 2011; 85(20): 10884–93. https://doi.org/10.1128/jvi.05265-11
- Neuber S., Wagner K., Goldner T., Lischka P., Steinbrueck L., Messerle M., et al. Mutual interplay between the human cytomegalovirus terminase subunits pUL51, pUL56, and pUL89 promotes terminase complex formation. J. Virol. 2017; 91(12): e02384-16. https://doi.org/10.1128/jvi.02384-16
- Piret J., Boivin G. Clinical development of letermovir and maribavir: Overview of human cytomegalovirus drug resistance. Antiviral. Res. 2019; 163: 91–105. https://doi.org/10.1016/j.antiviral.2019.01.011
- Lin A., Maloy M., Su Y., Bhatt V., DeRespiris L., Griffin M., et al. Letermovir for primary and secondary cytomegalovirus prevention in allogeneic hematopoietic cell transplant recipients: Real-world experience. Transpl. Infect. Dis. 2019; 21(6): 1–6. https://doi.org/10.1111/tid.13187
- Avery R.K., Alain S., Alexander B.D., Blumberg E.A., Chemaly R.F., Cordonnier C., et al. Maribavir for refractory cytomegalovirus infections with or without resistance post-transplant: results from a phase 3 randomized clinical trial. Clin. Infect. Dis. 2022; 75(4): 690–701. https://doi.org/10.1093/cid/ciab988
- Williams S.L., Hartline C.B., Kushner N.L., Harden E.A., Bidanset D.J., Drach J.C., et al. In vitro activities of benzimidazole D- and L-ribonucleosides against herpesviruses. Antimicrob. Agents Chemother. 2003; 47(7): 2186–92. https://doi.org/10.1128/aac.47.7.2186-2192.2003
- Shannon-Lowe C.D., Emery V.C. The effects of maribavir on the autophosphorylation of ganciclovir resistant mutants of the cytomegalovirus {UL}97 protein. Herpesviridae. 2010; 1(1): 4. https://doi.org/10.1186/2042-4280-1-4
- Sharma M., Bender B.J., Kamil J.P., Lye M.F., Pesola J.M., Reim N.I., et al. Human cytomegalovirus UL97 phosphorylates the viral nuclear egress complex. J. Virol. 2015; 89(1): 523–34. https://doi.org/10.1128/jvi.02426-14
- Chou S., Marousek G.I., Van Wechel L.C., Li S., Weinberg A. Growth and drug resistance phenotypes resulting from cytomegalovirus DNA polymerase region III mutations observed in clinical specimens. Antimicrob. Agents Chemother. 2007; 51(11): 4160–2. https://doi.org/10.1128/aac.00736-07
- O’Brien M.S., Markovich K.C., Selleseth D., DeVita A.V., Sethna P., Gentry B.G. In vitro evaluation of current and novel antivirals in combination against human cytomegalovirus. Antiviral. Res. 2018; 158: 255–63. https://doi.org/10.1016/j.antiviral.2018.08.015
- Chou S.W. Cytomegalovirus drug resistance and clinical implications. Transpl. Infect. Dis. 2001; 3(Suppl. 2): 20–4. https://doi.org/10.1034/j.1399-3062.2001.00004.x
- Jabs D.A., Enger C., Dunn J.P., Forman M., Hubbard L. Cytomegalovirus retinitis and viral resistance: 3. Culture results. Am. J. Ophthalmol. 1998; 126(4): 543–9. https://doi.org/10.1016/s0002-9394(98)00134-2
- Yu U., Wang X., Zhang X., Wang C., Yang C., Zhou X., et al. Cytomegalovirus infection and the implications of drug-resistant mutations in pediatric allogeneic hematopoietic stem cell transplant recipients: a retrospective study from a tertiary hospital in China. Infect. Dis. Ther. 2021; 10(3): 1309–22. https://doi.org/10.1007/s40121-021-00452-4
- Chou S. Approach to drug-resistant cytomegalovirus in transplant recipients. Curr. Opin. Infect. Dis. 2015; 28(4): 293–9. https://doi.org/10.1097/qco.0000000000000170
- Eckle T., Lang P., Prix L., Jahn G., Klingebiel T., Handgretinger R., et al. Rapid development of ganciclovir-resistant cytomegalovirus infection in children after allogeneic stem cell transplantation in the early phase of immune cell recovery. Bone Marrow Transplant. 2002; 30(7): 433–9. https://doi.org/10.1038/sj.bmt.1703666
- Wolf D.G., Yaniv I., Honigman A., Kassis I., Schonfeld T., Ashkenazi S. Early emergence of ganciclovir-resistant human cytomegalovirus strains in children with primary combined immunodeficiency. J. Infect. Dis. 1998; 178(2): 535–8. https://doi.org/10.1086/517468
- Littler E., Stuart A., Chee M. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992; 358(6382): 160–2. https://doi.org/10.1038/358160a0
- Kleiboeker S., Nutt J., Schindel B., Dannehl J., Hester J. Cytomegalovirus antiviral resistance: Characterization of results from clinical specimens. Transpl. Infect. Dis. 2014; 16(4): 561–7. https://doi.org/10.1111/tid.12241
- Chou S. Advances in the genotypic diagnosis of cytomegalovirus antiviral drug resistance. Antiviral. Res. 2020; 176: 104711. https://doi.org/10.1016/j.antiviral.2020.104711
- Campos A.B., Ribeiro J., Pinho Vaz C., Campilho F., Branca R., Campos A., et al. Genotypic resistance of cytomegalovirus to antivirals in hematopoietic stem cell transplant recipients from Portugal: A retrospective study. Antiviral. Res. 2017; 138: 86–92. https://doi.org/10.1016/j.antiviral.2016.10.016
- Chen H., Beardsley G.P., Coen D.M. Mechanism of ganciclovir-induced chain termination revealed by resistant viral polymerase mutants with reduced exonuclease activity. Proc. Natl Acad. Sci. USA. 2014; 111(49): 17462–7. https://doi.org/10.1073/pnas.1405981111
- Chou S., Marousek G.I. Accelerated evolution of maribavir resistance in a cytomegalovirus exonuclease domain II mutant. J. Virol. 2008; 82(1): 246–53. https://doi.org/10.1128/jvi.01787-07
- Houldcroft C.J., Bryant J.M., Depledge D.P., Margetts B.K., Simmonds J., Nicolaou S., et al. Detection of low frequency multi-drug resistance and novel putative maribavir resistance in immunocompromised pediatric patients with cytomegalovirus. Front. Microbiol. 2016; 7: 1317. https://doi.org/10.3389/fmicb.2016.01317
- Chou S., Satterwhite L.E., Ercolani R.J. New locus of drug resistance in the human cytomegalovirus UL56 gene revealed by in vitro exposure to letermovir and ganciclovir. Antimicrob. Agents Chemother. 2018; 62(9): e00922-18. https://doi.org/10.1128/aac.00922-18
- Agut H., Collandre H., Aubin J.T., Guétard D., Favier V., Ingrand D., et al. In vitro sensitivity of human herpesvirus-6 to antiviral drugs. Res. Virol. 1989; 140(3): 219–28. https://doi.org/10.1016/s0923-2516(89)80099-8
- Manichanh C., Grenot P., Gautheret-Dejean A., Debré P., Huraux J.M., Agut H. Susceptibility of human herpesvirus 6 to antiviral compounds by flow cytometry analysis. Cytometry. 2000; 40(2): 135–40. https://doi.org/10.1002/(sici)1097-0320(20000601)40:2%3C135::aid-cyto7%3E3.0.co;2-h
- De Clercq E., Naesens L., De Bolle L., Schols D., Zhang Y., Neyts J. Antiviral agents active against human. Rev. Med. Virol. 2001; 11(6): 381–95. https://doi.org/10.1002/rmv.336
- Kotton C.N., Kumar D., Caliendo A.M., Huprikar S., Chou S., Danziger-Isakov L., et al. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2018; 102(6): 900–31. https://doi.org/10.1097/tp.0000000000002191
- Sahoo M.K., Lefterova M.I., Yamamoto F., Waggoner J.J., Chou S., Holmes S.P., et al. Detection of cytomegalovirus drug resistance mutations by next-Generation sequencing. J. Clin. Microbiol. 2013; 51(11): 3700–10. https://doi.org/10.1128/jcm.01605-13
- Andrei G., Van Loon E., Lerut E., Victoor J., Meijers B., Bammens B., et al. Persistent primary cytomegalovirus infection in a kidney transplant recipient: Multi-drug resistant and compartmentalized infection leading to graft loss. Antiviral. Res. 2019; 168: 203–9. https://doi.org/10.1016/j.antiviral.2019.06.004
- De Bolle L., Naesens L., De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin. Microbiol. Rev. 2005; 18(1): 217–45. https://doi.org/10.1128/cmr.18.1.217-245.2005
- Humar A., Malkan G., Moussa G., Greig P., Levy G., Mazzulli T. Human herpesvirus-6 is associated with cytomegalovirus reactivation in liver transplant recipients. J. Infect. Dis. 2000; 181(4): 1450–3. https://doi.org/10.1086/315391
- Isegawa Y., Hara J., Amo K., Osugi Y., Takemoto M., Yamanishi K., et al. Human herpesvirus 6 ganciclovir-resistant strain with amino acid substitutions associated with the death of an allogeneic stem cell transplant recipient. J. Clin. Virol. 2009; 44(1): 15–9. https://doi.org/10.1016/j.jcv.2008.09.002
- Baldwin K. Ganciclovir-resistant Human herpesvirus-6 encephalitis in a liver transplant patient: A case report. J. Neurovirol. 2011; 17(2): 193–5. https://doi.org/10.1007/s13365-011-0019-4
- Bounaadja L., Piret J., Goyette N., Boivin G. Analysis of HHV-6 mutations in solid organ transplant recipients at the onset of cytomegalovirus disease and following treatment with intravenous ganciclovir or oral valganciclovir. J. Clin. Virol. 2013; 58(1): 279–82. https://doi.org/10.1016/j.jcv.2013.06.024
- Safronetz D., Petric M., Tellier R., Parvez B., Tipples G.A. Mapping ganciclovir resistance in the human herpesvirus-6 U69 protein kinase. J. Med. Virol. 2003; 71(3): 434–9. https://doi.org/10.1002/jmv.10510
Supplementary files