Исследование безопасности и иммуногенности вакцины на основе VLP для профилактики ротавирусной инфекции на модели новорожденных карликовых свиней
- Авторы: Костина Л.В.1, Филатов И.Е.1, Елисеева О.В.1, Латышев О.Е.1, Чернорыж Я.Ю.1, Юрлов К.И.1, Леснова Е.И.1, Хаметова К.М.1, Черепушкин С.А.1, Савочкина Т.Е.1, Цибезов В.В.1, Крышень К.Л.2, Алексеева Л.И.2, Зайкова О.Н.1, Гребенникова Т.В.1
-
Учреждения:
- ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
- АО «НПО «ДОМ ФАРМАЦИИ»
- Выпуск: Том 68, № 5 (2023)
- Страницы: 415-427
- Раздел: ОРИГИНАЛЬНЫЕ ИССЛЕДОВАНИЯ
- URL: https://journal-vniispk.ru/0507-4088/article/view/231860
- DOI: https://doi.org/10.36233/0507-4088-194
- EDN: https://elibrary.ru/bnjqgp
- ID: 231860
Цитировать
Полный текст
Аннотация
Введение. В России почти половина случаев острых кишечных инфекций установленной этиологии в 2022 г. приходится на ротавирусную инфекцию (РВИ). Специфического лечения ротавирусного гастроэнтерита не существует. Имеется необходимость создания современных эффективных и безопасных препаратов для борьбы с РВИ, не способных размножаться (реплицироваться) в организме вакцинируемого. Перспективным подходом является создание вакцин на основе вирусоподобных частиц (VLP).
Цель работы. Изучение безопасности и иммуногенности вакцины «Гам-VLP-рота» против РВИ на основе VLP ротавируса А человека на новорожденных карликовых свиньях при многократном внутримышечном введении.
Материалы и методы. В эксперименте были задействованы новорожденные карликовые свиньи. Безопасность тестируемой вакцины оценивали на основании данных термометрии, клинического осмотра, динамики массы тела, клинических и биохимических показателей крови, а также некропсии и гистологического исследования животных. Для оценки иммуногенности исследовали клеточный, гуморальный и секреторный иммунный ответ на введение вакцины.
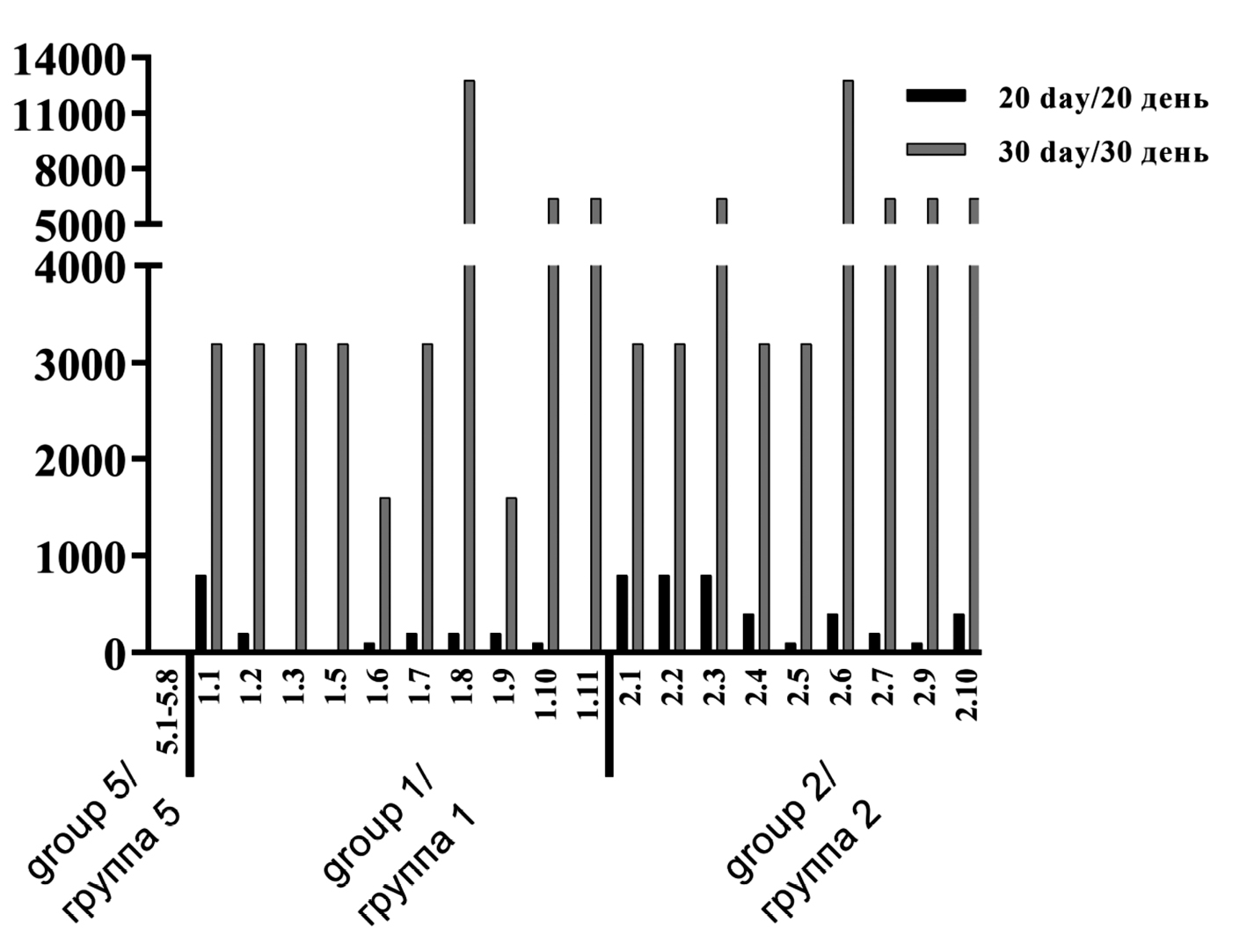
Результаты. Оценка общего состояния животных в период иммунизации, данные клинико-лабораторных и патоморфологических исследований свидетельствуют о безопасности вакцины «Гам-VLP-рота» при трехкратном внутримышечном введении. Установлена хорошая местная переносимость тестируемой вакцины. Формируется устойчивый гуморальный иммунитет после трехкратной вакцинации «Гам-VLP-рота», антиген-специфические IgG функционально активны в нейтрализации ротавируса А человека. Показано, что в минимальной исследуемой концентрации (30 мкг/доза) после трехкратной вакцинации у животных вырабатывался клеточно-опосредованный иммунный ответ. Полученные результаты титра IgA в сыворотке крови и в смывах кишечника свидетельствуют о формировании как системного иммунологического ответа, так и специфического секреторного иммунного ответа к ротавирусу А человека.
Заключение. Трехкратная внутримышечная иммунизация карликовых свиней вакциной «Гам-VLP-рота» формирует устойчивый защитный гуморальный, секреторный и клеточный иммунитет у исследуемых животных. Исследуемая вакцина безопасна и имеет хорошую местную переносимость.
Полный текст
Открыть статью на сайте журналаОб авторах
Людмила Владимировна Костина
ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
Автор, ответственный за переписку.
Email: lvkostina@mail.ru
ORCID iD: 0000-0002-9556-1454
кандидат биол. наук, старший научный сотрудник лаборатории молекулярной диагностики
Россия, 123098, МоскваИлья Евгеньевич Филатов
ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
Email: filat69rus@yandex.ru
ORCID iD: 0000-0001-5274-224X
младший научный сотрудник лаборатории молекулярной диагностики
Россия, 123098, МоскваОлеся Васильевна Елисеева
ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
Email: olesenka80@mail.ru
ORCID iD: 0000-0002-0723-9749
кандидат биол. наук, старший научный сотрудник лаборатории молекулярной диагностики
Россия, 123098, МоскваОлег Евгеньевич Латышев
ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
Email: oleglat80@mail.ru
ORCID iD: 0000-0002-5757-3809
кандидат биол. наук, заведующий лабораторией иммунологии
Россия, 123098, МоскваЯна Юрьевна Чернорыж
ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
Email: revengeful_w@mail.ru
ORCID iD: 0000-0001-9848-8515
кандидат медицинских наук, научный сотрудник
Россия, 123098, МоскваКирилл Иванович Юрлов
ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
Email: kir34292@yandex.ru
ORCID iD: 0000-0002-4694-2445
научный сотрудник
Россия, 123098, МоскваЕкатерина Ивановна Леснова
ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
Email: wolf252006@yandex.ru
ORCID iD: 0000-0002-2801-6843
научный сотрудник
Россия, 123098, МоскваКизхалум Маликовна Хаметова
ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
Email: kizkhalum@yandex.ru
ORCID iD: 0000-0002-8461-600X
кандидат биол. наук, научный сотрудник лаборатории средств специфической профилактики
Россия, 123098, МоскваСтанислав Андреевич Черепушкин
ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
Email: cherepushkin1@gmail.com
ORCID iD: 0000-0002-1734-5369
научный сотрудник лаборатории молекулярной диагностики
Россия, 123098, МоскваТатьяна Евгениевна Савочкина
ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
Email: tasavochkina@yandex.ru
ORCID iD: 0000-0003-4366-8476
младший научный сотрудник лаборатории молекулярной диагностики
Россия, 123098, МоскваВалерий Владимирович Цибезов
ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
Email: tsibezov@yandex.ru
ORCID iD: 0000-0003-2150-5764
кандидат биол. наук, ведущий научный сотрудник лаборатории средств специфической профилактики
Россия, 123098, МоскваКирилл Леонидович Крышень
АО «НПО «ДОМ ФАРМАЦИИ»
Email: kryshen.kl@doclinika.ru
ORCID iD: 0000-0003-1451-7716
кандидат биол. наук, руководитель отдела специфической токсикологии и микробиологии
Россия, 188663, Ленинградская обл., Всеволожский р-н, п. КузьмоловскийЛюбовь Игоревна Алексеева
АО «НПО «ДОМ ФАРМАЦИИ»
Email: alekseeva.li@doclinika.ru
ORCID iD: 0000-0002-6510-9897
младший научный сотрудник отдела специфической токсикологии и фармакодинамики, руководитель исследований
Россия, 188663, Ленинградская обл., Всеволожский р-н, п. КузьмоловскийОльга Николаевна Зайкова
ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
Email: zaykova_o_n@mail.ru
ORCID iD: 0000-0003-4708-2069
кандидат биологических наук, старший научный сотрудник лаборатории молекулярной диагностики, научный сотрудник
Россия, 123098, МоскваТатьяна Владимировна Гребенникова
ФГБУ «Национальный исследовательский центр эпидемиологии и микробиологии имени почетного академика Н.Ф. Гамалеи» Минздрава России
Email: t_grebennikova@mail.ru
ORCID iD: 0000-0002-6141-9361
доктор биол. наук, профессор, чл-корр. РАН, зав. лабораторией молекулярной диагностики, руководитель Испытательного центра, заместитель директора по науке
Россия, 123098, МоскваСписок литературы
- Hallowell B.D., Chavers T., Parashar U., Tate J.E. Global estimates of rotavirus hospitalizations among children below 5 years in 2019 and current and projected impacts of rotavirus vaccination. J. Pediatric Infect. Dis. Soc. 2022; 11(4): 149–58. https://doi.org/10.1093/jpids/piab114
- Troeger C., Khalil I.A., Rao P.C., Cao S., Blacker B.F., Ahmed T., et. al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018; 172(10): 958–65. https://doi.org/10.1001/jamapediatrics.2018.1960
- Государственный доклад «О состоянии санитарно-эпидемиологического благополучия населения в Российской Федерации в 2022 году». М.; 2023.
- Omatola C.A., Olaniran A.O. Rotaviruses: from pathogenesis to disease control – a critical review. Viruses. 2022; 14(5): 875. https://doi.org/10.3390/v14050875
- Vetter V., Gardner R.C., Debrus S., Benninghoff B., Pereira P. Established and new rotavirus vaccines: a comprehensive review for healthcare professionals. Hum. Vaccin. Immunother. 2022; 18(1): 1870395. https://doi.org/10.1080/21645515.2020.1870395
- Ivashechkin A.A., Yuzhakov A.G., Grebennikova T.V., Yuzhakova K.A., Kulikova N.Y., Kisteneva L.B., et al. Genetic diversity of group A rotaviruses in Moscow in 2018-2019. Arch. Virol. 2020; 165(3): 691–702. https://doi.org/10.1007/s00705-020-04534-5
- Yuzhakov A., Yuzhakova K., Kulikova N., Kisteneva L., Cherepushkin S., Smetanina S., et al. Prevalence and genetic diversity of group a rotavirus genotypes in Moscow (2019-2020). Pathogens. 2021; 10(6): 674. https://doi.org/10.3390/pathogens10060674
- Намазова-Баранова Л.С., Федосеенко М.В., Калюжная Т.А., Шахтахтинская Ф.Ч., Толстова С.В., Сельвян А.М. Новые возможности иммунопрофилактики ротавирусной инфекции в Российской Федерации. Обзор профиля инновационной ротавирусной вакцины. Педиатрическая фармакология. 2022; 19(6): 492–502. https://doi.org/10.15690/pf.v19i6.2489 https://elibrary.ru/zrbuqq
- Rotavirus vaccines: WHO position paper – July 2021. Wkly Epidemiol. Rec. 2021; 96(28): 301–20.
- Skansberg A., Sauer M., Tan M., Santosham M., Jennings M.C. Product review of the rotavirus vaccines ROTASIIL, ROTAVAC, and Rotavin-M1. Hum. Vaccin. Immunother. 2021; 17(4): 1223–34. https://doi.org/10.1080/21645515.2020.1804245
- Черепушкин С.А., Цибезов В.В., Южаков А.Г., Латышев О.Е., Алексеев К.П., Алтаева Е.Г. и др. Синтез и характеристика вирусоподобных частиц ротавируса А (Reoviridae: Sedoreovirinae: Rotavirus: Rotavirus A) человека. Вопросы вирусологии. 2021; 66(1): 55–64. https://doi.org/10.36233/0507-4088-27 https://elibrary.ru/eersag
- Директива 2010/63/EU. Европейского Парламента и Совета Европейского Союза по охране животных, используемых в научных целях. переулок с англ. СПб.; 2012: 1–48.
- Латышев О.Е., Елисеева О.В., Костина Л.В., Алексеев К.П., Хаметова К.М., Алтаева Е.Г. и др. Оценка иммуногенной активности клонированного штамма WA ротавируса А человека. Вопросы вирусологии. 2019; 64(4): 156–64. https://doi.org/10.36233/0507-4088-2019-64-4-156-164 https://elibrary.ru/sckbyy
- Филатов И.Е., Цибезов В.В., Баландина М.В., Норкина С.Н., Латышев О.Е., Елисеева О.В. и др. Использование вирусоподобных частиц на основе рекомбинантных вирусных белков VP2/VP6 ротавируса А для оценки гуморального иммунного ответа методом ИФА. Вопросы вирусологии. 2023; 68(2): 161–71. https://doi.org/10.36233/0507-4088-169 https://elibrary.ru/aywqhn
- Степанова О.И., Каркищенко В.Н., Клёсов Р.А., Станкова Н.В., Агельдинов Р.А., Савина М.А. Методика выделения лимфоидных клеток (мононуклеаров) из цельной крови, полученной от мини-свиньи. Биомедицина. 2020; 16(3): 54–9. https://doi.org/10.33647/2074-5982-16-3-54-59 https://elibrary.ru/ggmvjo
- Estes M.K., Crawford S.E., Penaranda M.E., Petrie B.L., Burns J.W., Chan W.K., et al. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J. Virol. 1987; 61(5): 1488–94. https://doi.org/10.1128/jvi.61.5.1488-1494.1987
- Li T., Lin H., Zhang Y., Li M., Wang D., Che Y., et al. Improved characteristics and protective efficacy in an animal model of E. coli-derived recombinant double-layered rotavirus virus-like particles. Vaccine. 2014; 32(17): 1921–31. https://doi.org/10.1016/j.vaccine.2014.01.093
- Rodríguez-Limas W.A., Tyo K.E., Nielsen J., Ramírez O.T., Palomares L.A. Molecular and process design for rotavirus-like particle production in Saccharomyces cerevisiae. Microb. Cell. Fact. 2011; 10: 33. https://doi.org/10.1186/1475-2859-10-33
- Kurokawa N., Lavoie P.O., D’Aoust M.A., Couture M.M., Dargis M., Trépanier S., et al. Development and characterization of a plant-derived rotavirus-like particle vaccine. Vaccine. 2021; 39(35): 4979–87. https://doi.org/10.1016/j.vaccine.2021.07.039
- Molinari P., Peralta A., Taboga O. Production of rotavirus-like particles in Spodoptera frugiperda larvae. J. Virol. Methods. 2008; 147(2): 364–7. https://doi.org/10.1016/j.jviromet.2007.09.002
- Lee J.M., Chung H.Y., Kim K.I., Yoo K.H., Hwang-Bo J., Chung I.S., et al. Synthesis of double-layered rotavirus-like particles using internal ribosome entry site vector system in stably-transformed Drosophila melanogaster. Biotechnol. Lett. 2011; 33(1): 41–6. https://doi.org/10.1007/s10529-010-0390-x
- Laura A., Palomares O.T.R. Challenges for the production of virus-like particles in insect cells: the case of rotavirus-like particles. Biochem. Eng. J. 2009; 45(3): 158–67. https://doi.org/10.1016/j.bej.2009.02.006
- Changotra H., Vij A. Rotavirus virus-like particles (RV-VLPs) vaccines: An update. Rev. Med. Virol. 2017; 27(6). https://doi.org/10.1002/rmv.1954
- Istrate C., Hinkula J., Charpilienne A., Poncet D., Cohen J., Svensson L., et al. Parenteral administration of RF 8-2/6/7 rotavirus-like particles in a one-dose regimen induce protective immunity in mice. Vaccine. 2008; 26(35): 4594–601. https://doi.org/10.1016/j.vaccine.2008.05.089
- Azevedo M., Vlasova A., Saif L. Human rotavirus virus-like particle vaccines evaluated in a neonatal gnotobiotic pig model of human rotavirus disease. Expert Rev. Vaccines. 2013; 12(2): 169–81. https://doi.org/10.1586/erv.13.3
- El-Attar L., Oliver S.L., Mackie A., Charpilienne A., Poncet D., Cohen J., et al. Comparison of the efficacy of rotavirus VLP vaccines to a live homologous rotavirus vaccine in a pig model of rotavirus disease. Vaccine. 2009; 27(24): 3201–8. https://doi.org/10.1016/j.vaccine.2009.03.043
- Kurokawa N., Robinson M.K., Bernard C., Kawaguchi Y., Koujin Y., Koen A., et al. Safety and immunogenicity of a plant-derived rotavirus-like particle vaccine in adults, toddlers and infants. Vaccine. 2021; 39(39): 5513–23. https://doi.org/10.1016/j.vaccine.2021.08.052
Дополнительные файлы