Простой высокочувствительный и специфичный серологический тест для выявления антител к вирусу Varicella-Zoster (Varicellovirus humanalpha3)
- Авторы: Нагиева Ф.Г.1, Баркова Е.П.1, Харченко О.С.1, Сидоров А.В.1, Алаторцева Г.И.1, Черепович Б.С.1, Тараканова Ю.Н.1, Трубачева О.А.1, Пашков Е.А.1,2, Ртищев А.А.1, Cвитич О.А.1,2, Зверев В.В.1,2
-
Учреждения:
- ФГБНУ «Научно-исследовательский институт вакцин и сывороток имени И.И. Мечникова»
- ФГАОУ ВО «Первый Московский государственный медицинский университет имени И.М. Сеченова» Минздрава России (Сеченовский Университет)
- Выпуск: Том 69, № 6 (2024)
- Страницы: 489-499
- Раздел: ОРИГИНАЛЬНЫЕ ИССЛЕДОВАНИЯ
- URL: https://journal-vniispk.ru/0507-4088/article/view/277911
- DOI: https://doi.org/10.36233/0507-4088-259
- EDN: https://elibrary.ru/ykzhop
- ID: 277911
Цитировать
Аннотация
Введение. Вирус ветряной оспы (ВО) и опоясывающего герпеса (Varicella-Zoster virus, VZV) – высококонтагиозный альфа-герпесвирус. Диагностика ВО остается сложной задачей, особенно в случаях ВО-прорыва, из-за трудностей диагностики на основе клинических симптомов, что обусловливает необходимость разработки надежных лабораторных тестов.
Цель. Разработка простого высокочувствительного и специфичного серологического теста для выявления антител к VZV в сыворотках крови человека и животных с помощью реакции пассивной гемагглютинации (РПГА).
Материалы и методы. Культуры клеток человека и животных; штаммы VZV; иммунные сыворотки человека и животных; моноклональные антитела к VZV. В РПГА использовали формализированные эритроциты баранов, кур и коз, сенсибилизированные вирусоспецифическими гликопротеинами (ГП) VZV из вируссодержащей жидкости.
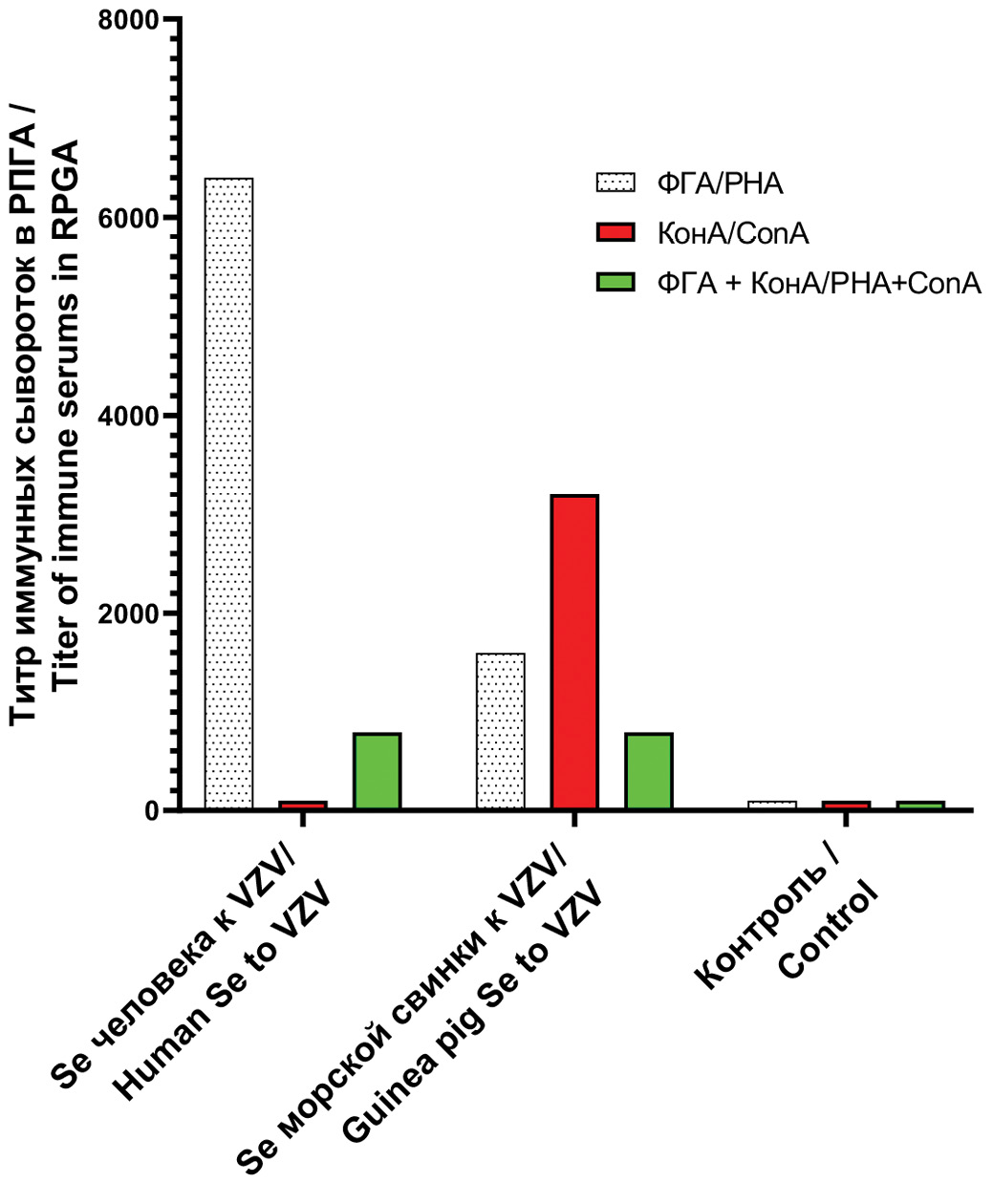
Результаты. Подобраны клеточные культуры с максимальным цитопатическим эффектом при заражении VZV. Разработан простой оригинальный метод получения вирусоспецифических ГП VZV с помощью лектинов. Очищенные ГП получены с помощью их элюирования при температуре 37 °С с бараньих эритроцитов после адсорбции при 4 °С. Активность ГП VZV подтверждена в РПГА на антительном диагностикуме, изготовленном путем сенсибилизации формализированных бараньих эритроцитов моноклональными антителами к ГП Е штамма «vОка» VZV (США). С применением ГП разных штаммов VZV разработаны тест-системы для выявления антител в иммунных сыворотках человека и животных методами РПГА и иммуноферментного анализ на основе ГП (gpИФА). Показаны высокая чувствительность, специфичность и отсутствие перекрестной реактивности этих тестов.
Заключение. Отобраны клеточные культуры c максимальным цитопатическим эффектом при заражении VZV. Разработан способ получения ГП из инфицированных клеток. С использованием очищенных вирусных ГП разработаны серологические тест-системы для выявления поствакцинальных и постинфекционных антител в иммунных сыворотках методами РПГА и gpИФА. Показаны высокая специфичность, чувствительность, воспроизводимость, а также простота их применения.
Полный текст
Открыть статью на сайте журналаОб авторах
Фирая Галиевна Нагиева
ФГБНУ «Научно-исследовательский институт вакцин и сывороток имени И.И. Мечникова»
Email: fgn42@yandex.ru
ORCID iD: 0000-0001-8204-4899
д-р мед. наук, доцент, заведующая лабораторией гибридных клеточных культур отдела вирусологии
Россия, 105064, г. МоскваЕлена Петровна Баркова
ФГБНУ «Научно-исследовательский институт вакцин и сывороток имени И.И. Мечникова»
Автор, ответственный за переписку.
Email: fgn42@yandex.ru
ORCID iD: 0000-0002-3369-8869
канд. биол. наук, ведущий научный сотрудник лаборатории гибридных клеточных культур отдела вирусологии
Россия, 105064, г. МоскваОльга Сергеевна Харченко
ФГБНУ «Научно-исследовательский институт вакцин и сывороток имени И.И. Мечникова»
Email: fgn42@yandex.ru
ORCID iD: 0000-0002-2169-9610
научный сотрудник лаборатории генетики ДНК-содержащих вирусов отдела вирусологии
Россия, 105064, г. МоскваАлександр Викторович Сидоров
ФГБНУ «Научно-исследовательский институт вакцин и сывороток имени И.И. Мечникова»
Email: fgn42@yandex.ru
ORCID iD: 0000-0002-3561-8295
канд. биол. наук, заведующий лабораторией генетики ДНК-содержащих вирусов отдела вирусологии
Россия, 105064, г. МоскваГалина Ивановна Алаторцева
ФГБНУ «Научно-исследовательский институт вакцин и сывороток имени И.И. Мечникова»
Email: fgn42@yandex.ru
ORCID iD: 0000-0001-9887-4061
канд. биол. наук, заведующая лабораторией клонирования вирусных геномов отдела вирусологии
Россия, 105064, г. МоскваБогдан Сергеевич Черепович
ФГБНУ «Научно-исследовательский институт вакцин и сывороток имени И.И. Мечникова»
Email: fgn42@yandex.ru
ORCID iD: 0000-0002-5803-6263
младший научный сотрудник лаборатории РНК-содержащих вирусов отдела вирусологии
Россия, 105064, г. МоскваЮлия Николаевна Тараканова
ФГБНУ «Научно-исследовательский институт вакцин и сывороток имени И.И. Мечникова»
Email: fgn42@yandex.ru
ORCID iD: 0000-0003-3226-5989
канд. биол. наук, заведующая лабораторией диагностики вирусных инфекций отдела вирусологии
Россия, 105064, г. МоскваОльга Анатольевна Трубачева
ФГБНУ «Научно-исследовательский институт вакцин и сывороток имени И.И. Мечникова»
Email: fgn42@yandex.ru
ORCID iD: 0009-0005-0821-5553
ведущий специалист лаборатории гибридных клеточных культур отдела вирусологии
Россия, 105064, г. МоскваЕвгений Алексеевич Пашков
ФГБНУ «Научно-исследовательский институт вакцин и сывороток имени И.И. Мечникова»; ФГАОУ ВО «Первый Московский государственный медицинский университет имени И.М. Сеченова» Минздрава России (Сеченовский Университет)
Email: fgn42@yandex.ru
ORCID iD: 0000-0002-5682-4581
младший научный сотрудник лаборатории прикладной вирусологии отдела вирусологии
Россия, 105064, г. Москва; 119048, г. МоскваАртем Андреевич Ртищев
ФГБНУ «Научно-исследовательский институт вакцин и сывороток имени И.И. Мечникова»
Email: fgn42@yandex.ru
ORCID iD: 0000-0002-4212-5093
научный сотрудник лаборатории генетики РНК-содержащих вирусов отдела вирусологии
Россия, 105064, г. МоскваОксана Анатольевна Cвитич
ФГБНУ «Научно-исследовательский институт вакцин и сывороток имени И.И. Мечникова»; ФГАОУ ВО «Первый Московский государственный медицинский университет имени И.М. Сеченова» Минздрава России (Сеченовский Университет)
Email: fgn42@yandex.ru
ORCID iD: 0000-0003-1757-8389
д-р мед. наук, член-корр. РАН, директор
Россия, 105064, г. Москва; 119048, г. МоскваВиталий Васильевич Зверев
ФГБНУ «Научно-исследовательский институт вакцин и сывороток имени И.И. Мечникова»; ФГАОУ ВО «Первый Московский государственный медицинский университет имени И.М. Сеченова» Минздрава России (Сеченовский Университет)
Email: fgn42@yandex.ru
ORCID iD: 0000-0001-5808-2246
д-р биол. наук, профессор, академик РАН, научный руководитель
Россия, 105064, г. Москва; 119048, г. МоскваСписок литературы
- Gershon A.A., Breuer J., Cohen J.I., Cohrs R.J., Gershon M.D., Gilden D., et al. Varicella zoster virus infection. Nat. Rev. Dis. Primers. 2015; 1: 15016. https://doi.org/10.1038/nrdp.2015.16
- Arvin A.M., Moffat J.F., Abendroth A., Oliver S.L., eds. Varicella-zoster Virus. Genetics, Pathogenesis and Immunity. 6th ed. Cham: Springer; 2023. https://doi.org/10.1007/978-3-031-15305-1
- Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec. 2014; 89(25): 265–87.
- Heininger U., Seward J.F. Varicella. Lancet. 2006; 368(9544): 1365–76. https://doi.org/10.1016/S0140-6736(06)69561-5
- Harpaz R., Ortega-Sanchez I.R., Seward J.F. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2008; 57(RR-5): 1–30; quiz CE2-4.
- Cohen J.I. Clinical practice: Herpes zoster. N. Engl. J. Med. 2013; 369(3): 255–63. https://doi.org/10.1056/NEJMcp1302674
- Shin D., Shin Y., Kim E., Nam H., Nan H., Lee J. Immunological characteristics of MAV/06 strain of varicella-zoster virus vaccine in an animal model. BMC Immunol. 2022; 23(1): 27. https://doi.org/10.1186/s12865-022-00503-6
- Higashimoto Y., Hattori F., Kawamura Y., Kozawa K., Hamano A., Kato M., et al. Analysis of the reliability of rapid diagnostic tests for varicella, including breakthrough cases. J. Med. Virol. 2023; 95(2): e28569. https://doi.org/10.1002/jmv.28569
- Pan D., Wang W., Cheng T. Current methods for the detection of antibodies of varicella-zoster virus: a review. Microorganisms. 2023; 11(2): 519. https://doi.org/10.3390/microorganisms11020519
- Otani N., Shima M., Tanimura S., Ueda T., Ichiki K., Nakajima K., et al. Sensitivity and specificity of different antibody tests for detecting varicella-zoster virus. J. Infect. Chemother. 2020; 26(12): 1283–7. https://doi.org/10.1016/j.jiac.2020.07.012
- Mo C., Lee J., Sommer M., Grose C., Arvin A.M. The requirement of varicella zoster virus glycoprotein E (gE) for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology. 2002; 304(2): 176–86. https://doi.org/10.1006/viro.2002.1556
- Berarducci B., Rajamani J., Reichelt M., Sommer M., Zerboni L., Arvin A.M. Deletion of the first cysteine-rich region of the varicella-zoster virus glycoprotein E ectodomain abolishes the gE and gI interaction and differentially affects cell-cell spread and viral entry. J. Virol. 2009; 83(1): 228–40. https://doi.org/10.1128/JVI.00913-08
- Hwang J.Y., Kim Y., Lee K.M., Shin O.S., Gim J.A., Shin Y., et al. Cross-reactive humoral immunity of clade 2 Oka and MAV/06 strain-based varicella vaccines against different clades of varicella-zoster virus. Hum. Vaccin. Immunother. 2023; 19(1): 2210961. https://doi.org/10.1080/21645515.2023.2210961
- Marin M., Güris D., Chaves S.S., Schmid S., Seward J.F. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2007; 56(RR-4): 1–40.
- Сenters for Disease Control and Prevention. Chickenpox (Varicella). Available at: https://cdc.gov/chickenpox/hcp/index.html
- Lafreniere M.A., Badr E., Beattie J., Macri J., Khan W.I. Performance evaluation system of the Bio–Rad Bioplex 2200 multiplex system in the detection of measles, mumps, rubella, and varicella-zoster antibodies. J. Clin. Virol. Plus. 2023; 3(1): 100131. https://doi.org/10.1016/j.jcvp.2022.100131and
- Coates S.R., Madsen R.D., Rippe D.F. New passive hemagglutination assay kit that uses hemagglutinin-sensitized erythrocytes for detection of rubella antibodies. J. Clin. Microbiol. 1982; 16(6): 1117–22. https://doi.org/10.1128/jcm.16.6.1117-1122.1982
- Kim K.S., Sapienza V., Chen C.M. Confirmation of human cytomegalovirus by reverse passive hemagglutination with monoclonal antibodies reactive to the major glycosylated peptide (GP-66). J. Clin. Microbiol. 1986; 24(3): 474–7. https://doi.org/10.1128/jcm.24.3.474-477.1986
- Maduike C.O., Ezeibe A.A., Anene N.I., Amechi B., Eze J.I., Animoke P.C. Direct passive hemagglutination test for rapid quantification of plasma load of the human immunodeficiency virus. Sci. Res. 2013; 5(9): 1351–4. http://dx.doi.org/10.4236/health.2013.59183
- Wasmuth E.H., Miller W.J. Sensitive enzyme-linked immunosorbent assay for antibody to varicella-zoster virus using purified VZV glycoprotein antigen. J. Med. Virol. 1990; 32(3): 189–93. https://doi.org/10.1002/jmv.1890320310
- Kino Y., Minamishima Y. Passive hemagglutination assays for the detection of antibodies to herpes viruses. Microbiol. Immunol. 1993; 37(5): 365–8. https://doi.org/10.1111/j.1348-0421.1993.tb03223.x
- Weinbach R. Die Verwendbarkeit formolbehandelter Erythocyten als Antigtntrager in der Haemagglutination. Schweiz. Z. Pathol. Bakteriol. 1958; 21(6): 1043–52. https://doi.org/10.1159/000160565 (in German)
- Фриго Н.В., Комарова В.Д., Обрядина А.П., Бурков А.Н. Сравнительные результаты иммуноферментного анализа, реакции пассивной гемагглютинации и микрореакции в серодиагностике сифилиса. Вестник дерматологии и венерологии. 2000; (4): 4–36.
- Mendelson E., Aboudy Y., Smetana Z., Tepperberg M., Grossman Z. Laboratory assessment and diagnosis of congenital viral infections: Rubella, cytomegalovirus (CMV), varicella-zoster virus (VZV), herpes simplex virus (HSV), parvovirus B19 and human immunodeficiency virus (HIV). Reprod. Toxicol. 2006; 21(4): 350–82. https://doi.org/10.1016/j.reprotox.2006.02.001
- Нагиева Ф.Г., Баркова Е.П., Лисаков А.Н., Сидоров А.В., Зверев В.В., Осокина О.В. и др. Практические аспекты выявления, культивирования и характеристики клинических изолятов вируса varicella-zoster. Инфекция и иммунитет. 2020; 10(2): 387–96. https://doi.org/10.15789/2220-7619-PAO-1211 https://elibrary.ru/ptnvte
- Шубладзе А.К., Гайдамович С.Я. Краткий курс практической вирусологии. М.: Медгиз; 1954.
Дополнительные файлы