Genetic diversity of parechoviruses (Picornaviridae: Paavivirinae: Parechovirus: Parechovirus ahumpari) circulating in Nizhny Novgorod in 2021–2024
- Authors: Zverev V.V.1, Selivanova S.G.1, Epifanova N.V.1, Kashnikov A.Y.1, Ponomareva N.V.1, Golitsyna L.N.1, Novikova N.A.1
-
Affiliations:
- Academician I.N. Blokhina Nizhny Novgorod Research Institute of Epidemiology and Microbiology, Rospotrebnadzor
- Issue: Vol 70, No 6 (2025)
- Pages: 581-588
- Section: ORIGINAL RESEARCHES
- URL: https://journal-vniispk.ru/0507-4088/article/view/375507
- DOI: https://doi.org/10.36233/0507-4088-351
- EDN: https://elibrary.ru/vyskys
- ID: 375507
Cite item
Abstract
Introduction. Parechoviruses of the Parechovirus ahumpari (PeV-A) species, pathogenic to humans, are widespread and genetically diverse infectious agents. Infections caused by these viruses are characterized by a wide variety of clinical manifestations ranging from mild intestinal or respiratory diseases to severe CNS lesions. The high-risk group for the disease are newborns and infants. PeV-A species are classified in 19 types that have a varying distribution in different territories. In Russia, the type composition of territorial parechovirus populations has not been sufficiently studied, which determines the relevance of monitoring the circulation of these viruses using genotyping.
The aim of the study was to identify and investigate the genetic diversity of parechoviruses that circulated in Nizhny Novgorod in the period 2021–2024.
Materials and methods. 5,073 stool samples from children hospitalized in an infectious hospital with a diagnosis of acute gastroenteritis were examined for the presence of human parechoviruses. The detection of parechoviruses was carried out by RT-PCR. Viral types were determined by Sanger sequencing of VP1 genome fragment. The nucleotide sequences were analyzed using MEGA X and Beast v1.8.4 software.
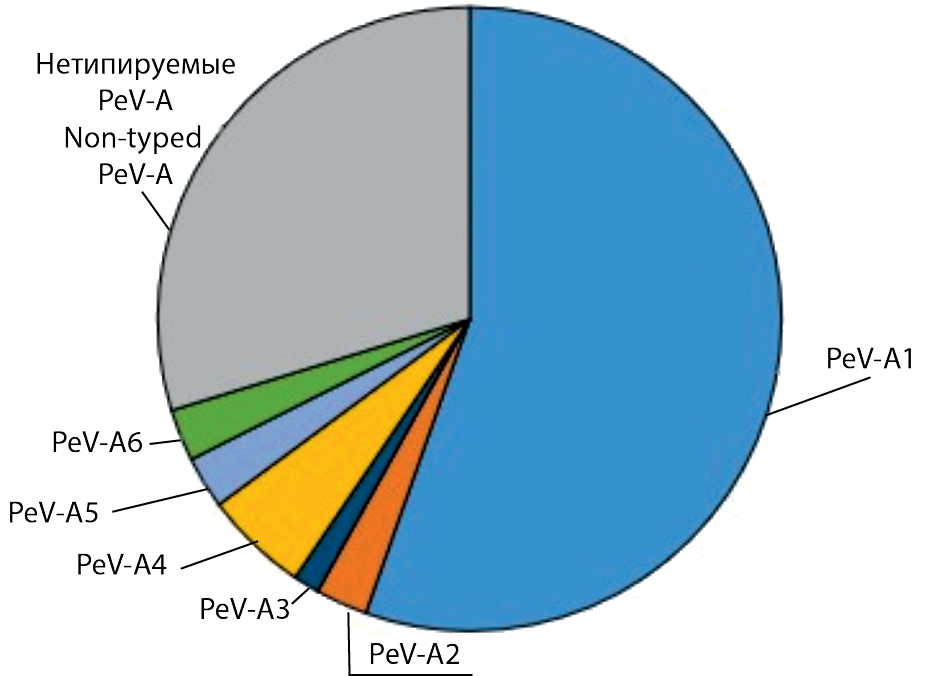
Results. Parechoviruses were detected in children aged 3 months to 17 years with a frequency of 0.06–2.08% in different years, an average of 1.46 ± 0.16%. Viral type has been identified for 52 strains. Six types of PeV-A parechoviruses have been identified. The PeV-A1 was a predominant type (80.4%). Types PeV-A2 to PeV-A6 have been found in isolated cases. Heterogeneity of the PeV-A1 population in Nizhny Novgorod was represented by virus genotypes 1A and 1B, with an absolute predominance of genotype 1B, which included 16 genetic variants.
Conclusion. The data obtained expand information on the type and genetic diversity of pathogenic for humans parechoviruses circulating among the population of central Russia (based the example of the Nizhny Novgorod region).
Keywords
About the authors
Vladimir V. Zverev
Academician I.N. Blokhina Nizhny Novgorod Research Institute of Epidemiology and Microbiology, Rospotrebnadzor
Email: arceo@yandex.ru
ORCID iD: 0000-0002-3853-9293
PhD (Biology), Senior Researcher, Laboratory of Molecular Epidemiology of Viral Infections
Russian Federation, 603950, Nizhny NovgorodSvetlana G. Selivanova
Academician I.N. Blokhina Nizhny Novgorod Research Institute of Epidemiology and Microbiology, Rospotrebnadzor
Email: svetafor22@mail.ru
ORCID iD: 0000-0002-6610-1774
PhD (Biology), Senior Researcher, Laboratory of Molecular Epidemiology of Viral Infections
Russian Federation, 603950, Nizhny NovgorodNatalia V. Epifanova
Academician I.N. Blokhina Nizhny Novgorod Research Institute of Epidemiology and Microbiology, Rospotrebnadzor
Email: epifanovanv@mail.ru
ORCID iD: 0000-0001-7679-8029
PhD (Biology), Leading Researcher, Laboratory of Molecular Epidemiology of Viral Infections
Russian Federation, 603950, Nizhny NovgorodAlexander Y. Kashnikov
Academician I.N. Blokhina Nizhny Novgorod Research Institute of Epidemiology and Microbiology, Rospotrebnadzor
Email: mevirfc@mail.ru
ORCID iD: 0000-0003-1033-7347
Researcher, Laboratory of Molecular Epidemiology of Viral Infections
Russian Federation, 603950, Nizhny NovgorodNatalia V. Ponomareva
Academician I.N. Blokhina Nizhny Novgorod Research Institute of Epidemiology and Microbiology, Rospotrebnadzor
Email: natalia.ponomareva.rfc@gmail.com
ORCID iD: 0000-0001-8950-6259
PhD (Biology), Senior Researcher, Laboratory of Molecular Epidemiology of Viral Infections
Russian Federation, 603950, Nizhny NovgorodLyudmila N. Golitsyna
Academician I.N. Blokhina Nizhny Novgorod Research Institute of Epidemiology and Microbiology, Rospotrebnadzor
Email: lyudmila_galitzina@mail.ru
ORCID iD: 0000-0002-8064-4476
PhD (Biology), Leading Researcher, Laboratory of Molecular Epidemiology of Viral Infections
Russian Federation, 603950, Nizhny NovgorodNadezhda A. Novikova
Academician I.N. Blokhina Nizhny Novgorod Research Institute of Epidemiology and Microbiology, Rospotrebnadzor
Author for correspondence.
Email: novikova_na@mail.ru
ORCID iD: 0000-0002-3710-6648
Doctor of Biological Sciences, Professor, Head of the Laboratory of Molecular Epidemiology of Viral Infections, Leading Researcher
Russian Federation, 603950, Nizhny NovgorodReferences
- Hyypiä T., Horsnell C., Maaronen M., Khan M., Kalkkinen N., Auvinen P., et al. A distinct picornavirus group identified by sequence analysis. Proc. Natl. Acad. Sci. USA. 1992; 89(18): 8847–51. https://doi.org/10.1073/pnas.89.18.8847
- Wigand R., Sabin A.B. Properties of ECHO types 22, 23 and 24 viruses. Arch. Gesamte. Virusforsch. 1961; 11: 224–47. https://doi.org/10.1007/BF01241688
- Stanway G., Kalkkinen N., Roivainen M., Ghazi F., Khan M., Smyth M., et al. Molecular and biological characteristics of echovirus 22, a representative of a new picornavirus group. J. Virol. 1994; 68(12): 8232–8. https://doi.org/10.1128/JVI.68.12.8232-8238.1994
- Zell R., Delwart E., Gorbalenya A.E., Hovi T., King A.M.Q., Knowles N.J., et al. ICTV virus taxonomy profile: Picornaviridae. J. Gen. Virol. 2017; 98(10): 2421–2. https://doi.org/10.1099/jgv.0.000911
- Stanway G., Joki-Korpela P., Hyypiä T. Human parechoviruses – biology and clinical significance. Rev. Med. Virol. 2000; 10(1): 57–69. https://doi.org/10.1002/(sici)1099-1654(200001/02)10:1<57::aid-rmv266>3.0.co;2-h
- Verboon-Maciolek M.A., Groenendaal F., Hahn C.D., Hellmann J., van Loon A.M., Boivin G., et al. Human parechovirus causes encephalitis with white matter injury in neonates. Ann. Neurol. 2008; 64(3): 266–73. https://doi.org/10.1002/ana.21445
- Benschop K.S., Schinkel J., Minnaar R.P., Pajkrt D., Spanjerberg L., Kraakman H.C., et al. Human parechovirus infections in Dutch children and the association between serotype and disease severity. Clin. Infect. Dis. 2006; 42(2): 204–10. https://doi.org/10.1086/498905
- Kabuga A.I., Nejati A., Soheili P., Shahmahmoodi S. Human parechovirus are emerging pathogens with broad spectrum of clinical syndromes in adults. J. Med. Virol. 2020; 92(12): 2911–6. https://doi.org/10.1002/jmv.26395
- Brouwer L., Wolthers K.C., Pajkrt D. Parechovirus A prevalence in adults in The Netherlands. Arch. Virol. 2020; 165(4): 963–6. https://doi.org/10.1007/s00705-020-04547-0
- Alam F., Li Y., Vogt M.R. Parechovirus: neglected for too long? J. Virol. 2025; 99(4): e0184624. https://doi.org/10.1128/jvi.01846-24
- Golitsyna L.N., Zverev V.V., Novikova N.A., Fomina S.G., Epifanova N.V., Lukovnikova L.B. Detection of parechoviruses in children with gastroenteritis. In: Proceedings of the IX Congress of the All-Russian Scientific and Practical Society of Epidemiologists, Microbiologists and Parasitologists. Volume 2 [Materialy IX s”ezda Vserossiiskogo nauchno-prakticheskogo obshchestva epidemiologov, mikrobiologov i parazitologov. Tom 2]. Moscow; 2007: 224–5. https://elibrary.ru/vyveqn (in Russian)
- Golitsyna L.N., Zverev V.V., Novikova N.A., Fomina S.G., Parfenova O.V., Epifanova N.V., et al. Prevalence, features of circulation, and diversity of human parechoviruses in Nizhny Novgorod. Voprosy virusologii. 2013; 58(2): 29–33. https://elibrary.ru/pzlptj (in Russian)
- Zhirakovskaia E., Tikunov A., Babkin I., Tikunova N. Complete genome sequences of the first parechoviruses A associated with sporadic pediatric acute gastroenteritis in Russia. Infect. Genet. Evol. 2020; 80: 104214. https://doi.org/10.1016/j.meegid.2020.104214
- Nix W.A., Maher K., Pallansch M.A., Oberste M.S. Parechovirus typing in clinical specimens by nested or semi-nested PCR coupled with sequencing. J. Clin. Virol. 2010; 48(3): 202–7. https://doi.org/10.1016/j.jcv.2010.04.007
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018; 35(6): 1547–9. https://doi.org/10.1093/molbev/msy096
- Drummond A.J., Suchard M.A., Xie D., Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012; 29(8): 1969–73. https://doi.org/10.1093/molbev/mss075
- Benschop K.S., de Vries M., Minnaar R.P., Stanway G., van der Hoek L., Wolthers K.C., et al. Comprehensive full-length sequence analyses of human parechoviruses: diversity and recombination. J. Gen. Virol. 2010; 91(Pt. 1): 145–54. https://doi.org/10.1099/vir.0.014670-0
- Golitsyna L.N., Zverev V.V., Novikova N.A. Human Parechoviruses [Parekhovirusy cheloveka]. Nizhny Novgorod: Rastr-NN; 2017. https://elibrary.ru/zgmsgr (in Russian)
- Mizuta K., Itagaki T., Katsushima F., Katsushima Y., Sasaki M., Komabayashi K., et al. Longitudinal antigenic and seroepidemiological analyses of parechovirus A1 in Yamagata, Japan. J. Med. Virol. 2023; 95(4): e28696. https://doi.org/10.1002/jmv.28696
- Li W., Gao Z., Yan H., Tian Y., Liu B., Shen L., et al. Prevalence and genetic diversity of Parechovirus A in children with diarrhea in Beijing, China, 2017–2019. Infect. Genet. Evol. 2023; 111: 105435. https://doi.org/10.1016/j.meegid.2023.105435
- Elling R., Böttcher S., du Bois F., Müller A., Prifert C., Weissbrich B., et al. Epidemiology of human Parechovirus type 3 upsurge in 2 hospitals, Freiburg, Germany, 2018. Emerg. Infect. Dis. 2019; 25(7): 1384–8. https://doi.org/10.3201/eid2507.190257
- Bubba L., Broberg E.K., Fischer T.K., Simmonds P., Harvala H. Parechovirus A circulation and testing capacities in Europe, 2015–2021. Emerg. Infect. Dis. 2024; 30(2): 234–44. https://doi.org/10.3201/eid3002.230647
- Tao L., Humphries R.M., Banerjee R., Gaston D.C. Re-emergence of Parechovirus: 2017–2022 national trends of detection in cerebrospinal fluid. Open Forum Infect. Dis. 2023; 10(3): ofad112. https://doi.org/10.1093/ofid/ofad112
- Wang C.Y.T., Ware R.S., Lambert S.B., Mhango L.P., Tozer S., Day R., et al. Parechovirus A infections in healthy Australian children during the first 2 years of life: a community-based longitudinal birth cohort study. Clin. Infect. Dis. 2020; 71(1): 116–27. https://doi.org/10.1093/cid/ciz761
- Sridhar A., Karelehto E., Brouwer L., Pajkrt D., Wolthers K.C. Parechovirus A pathogenesis and the enigma of genotype A-3. Viruses. 2019; 11(11): 1062. https://doi.org/10.3390/v11111062
Supplementary files