Herd immunity to SARS-CoV-2 in the Novosibirsk Region population amid the COVID-19 pandemic
- Authors: Popova A.Y.1, Smirnov V.S.2, Ezhlova E.B.1, Mel’nikova A.A.1, Samoilova L.V.3, Lyalina L.V.2, Semenova E.V.4, Gurskiy M.A.4, Aksenova E.A.5, Arbuzova T.V.2, Totolian A.A.2
-
Affiliations:
- Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
- FBSI «Saint Petersburg Pasteur Research Institute of Epidemiology and Mictobiology» of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
- Administration of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor) for the Novosibirsk Region
- FBIH «Center for Hygiene and Epidemiology in the Novosibirsk Region»
- Ministry of Health of the Novosibirsk Region
- Issue: Vol 66, No 4 (2021)
- Pages: 299-309
- Section: ORIGINAL RESEARCHES
- URL: https://journal-vniispk.ru/0507-4088/article/view/118193
- DOI: https://doi.org/10.36233/0507-4088-54
- ID: 118193
Cite item
Full Text
Abstract
Aim. To determine the level of SARS-CoV-2 seroprevalence among the Novosibirsk Region population against the background of the COVID-19 pandemic.
Material and methods. The work was carried out in 2 phases: 1) a cross-sectional cohort study performed 28.06– 15.07.2020; 2) longitudinal cohort 3-stage seromonitoring: 1st stage 28.06–15.07.2020; 2nd 14.09–04.10.2020; 3rd 10–30.12.2020 The work was carried out according to a unified methodology developed by Rospotrebnadzor with the participation of St-Petersburg Pasteur Institute, taking into account the recommendations of the WHO. IgG antibodies to the SARS-CoV-2 nucleocapsid protein were detected by ELISA using a kit of reagents produced by the SRCMSB (Obolensk) according to the manufacturer’s instructions. Statistical analysis was performed using Microsoft Excel 2010 and other programs.
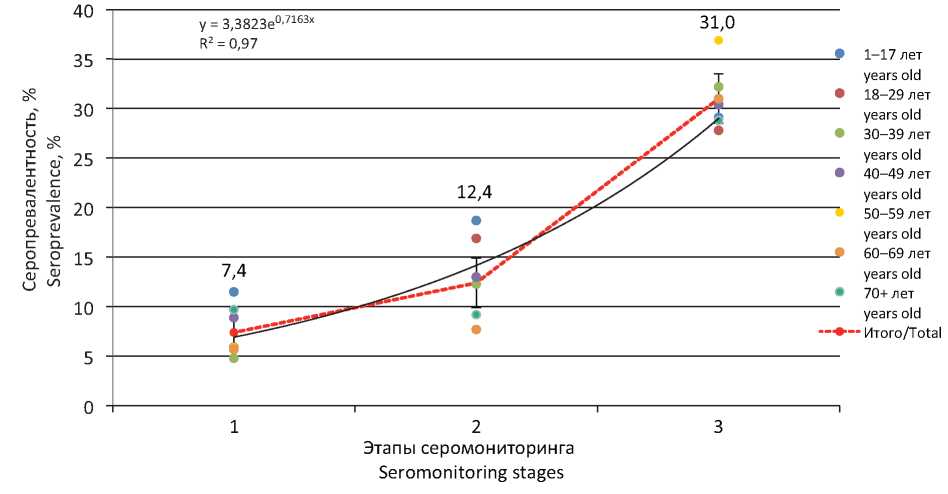
Results. The seroprevalence in the region’s population was 9.1% (95% CI 8.0–10.2): maximum in children 14–17 years old (17.6%, 95% CI 12.3–23.9) and persons over 75 years (14.8%, 95% CI 11.4–18.8), minimum among persons 30–39 years old (4.9%, 95% CI 3.0–8.0). Increased rate was noted among the unemployed (15.4%, 95% CI 9.9–17.1) and other individuals (13.0%, 95% CI 8.6–18.5). Seroprevalence was 33.3% (95% CI 16.3–59.0) in COVID-19 convalescents and 19.0% (95% CI 13.9-25.0) in contact persons. More than 94.7% (95% CI 91.2–97.2) of seropositive individuals were asymptomatic. During the serological monitoring, seroprevalence increased from 7.4% (95% CI 6.2–8.9) at 1st stage 1 to 12.4% (95% CI 10.6–14.3) at 2nd , and 31% (95% CI 28.8–33.3) at 3rd stage.
Conclusion. SARS-CoV-2 herd immunity has not reached the threshold level, this does not exclude exacerbation of the epidemic process.
Keywords
Full Text
##article.viewOnOriginalSite##About the authors
A. Yu. Popova
Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
Email: fake@neicon.ru
ORCID iD: 0000-0003-2567-9032
127994, Moscow, Russia
Russian FederationV. S. Smirnov
FBSI «Saint Petersburg Pasteur Research Institute of Epidemiology and Mictobiology» of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
Author for correspondence.
Email: vssmi@mail.ru
ORCID iD: 0000-0002-2723-1496
Vyacheslav S. Smirnov, D.Sci. (Med.), Professor, Leading Researcher of the Laboratory of Molecular Immunology
197101, Saint Petersburg, Russia
Russian FederationE. B. Ezhlova
Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
Email: fake@neicon.ru
ORCID iD: 0000-0002-8701-280X
127994, Moscow, Russia
Russian FederationA. A. Mel’nikova
Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
Email: fake@neicon.ru
ORCID iD: 0000-0002-5651-1331
127994, Moscow, Russia
Russian FederationL. V. Samoilova
Administration of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor) for the Novosibirsk Region
Email: fake@neicon.ru
ORCID iD: 0000-0003-4836-9010
630132, Novosibirsk, Russia
Russian FederationL. V. Lyalina
FBSI «Saint Petersburg Pasteur Research Institute of Epidemiology and Mictobiology» of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
Email: fake@neicon.ru
ORCID iD: 0000-0001-9921-3505
197101, Saint Petersburg, Russia
Russian FederationE. V. Semenova
FBIH «Center for Hygiene and Epidemiology in the Novosibirsk Region»
Email: fake@neicon.ru
ORCID iD: 0000-0002-7715-7036
630099, Novosibirsk, Russia
Russian FederationM. A. Gurskiy
FBIH «Center for Hygiene and Epidemiology in the Novosibirsk Region»
Email: fake@neicon.ru
ORCID iD: 0000-0002-5951-0940
630099, Novosibirsk, Russia
Russian FederationE. A. Aksenova
Ministry of Health of the Novosibirsk Region
Email: fake@neicon.ru
ORCID iD: 0000-0001-6515-2169
630007, Novosibirsk, Russia
Russian FederationT. V. Arbuzova
FBSI «Saint Petersburg Pasteur Research Institute of Epidemiology and Mictobiology» of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
Email: fake@neicon.ru
ORCID iD: 0000-0002-3074-8656
197101, Saint Petersburg, Russia
Russian FederationA. A. Totolian
FBSI «Saint Petersburg Pasteur Research Institute of Epidemiology and Mictobiology» of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
Email: fake@neicon.ru
ORCID iD: 0000-0003-4571-8799
197101, Saint Petersburg, Russia
Russian FederationReferences
- WHO. Disease outbreak news – China. Available at: https://www.who.int/ru/emergencies/disease-outbreak-news/item/2020-DON229 (accessed August 12, 2021).
- Novel Coronavirus(2019-nCoV). Situation Report - 10. Available at: https://www.who.int/docs/default-source/coronaviruse/situationreports/20200130-sitrep-10-ncov.pdf (accessed August 12, 2021).
- Holshue M.L., DeBolt C., Lindquist S, Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020; 382(10): 929–36. https://doi.org/10.1056/nejmoa2001191
- WHO. Director-General’s remarks at 2019-nCoV coronavirus infection press briefing, 11 February 2020. Available at: https://www.who.int/ru/dg/speeches/detail/who-director-general-s-remarks-atthe-media-briefing-on-2019-ncov-on-11-february-2020 (accessed August 12, 2021).
- Коронавирус. Онлайн карта распространения коронавируса. Коронавирус в России и мире. Available at: https://www.coronavirus-monitor.ru (accessed August 12, 2021).
- Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020; 20(6): 363–74. https://doi.org/10.1038/s41577-020-0311-8
- Ni L., Ye F., Cheng M.L., Feng Y., Deng Y.Q., Zhao H., et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020; 52(6): 971–7. https://doi.org/10.1016/j.immuni.2020.04.023
- Hou H., Wang T., Zhang B., Luo Y., Mao L., Wang F., et al. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin. Transl. Immunology. 2020; 9(5): e01136. https://doi.org/10.1002/cti2.11363
- Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor- Phillips S., et al. Cochrane COVID-19 diagnostic test accuracy group. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020; 6(6): CD013652. https://doi.org/10.1002/14651858.CD013652
- Randolph H.E., Barreiro L.B. Herd immunity: Understanding COVID-19. Immunity. 2020; 52(5): 737–41. https://doi.org/10.1016/j.immuni.2020.04.012
- Clemente-Suárez V.J., Hormeño-Holgado A., Jiménez M., Benitez- Agudelo J.C., Navarro-Jiménez E., Perez-Palencia N., et al. Dynamics of Population Immunity Due to the Herd Effect in the COVID-19 Pandemic. Vaccines (Basel). 2020; 8(2): E236. https://doi.org/10.3390/vaccines8020236
- Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., et al. Dynamics and significance of the antibody response to SARS-CoV-2 infection. medRxiv. 2020; 5(52): eabe0367. https://doi.org/10.1101/2020.07.18.20155374
- Попова А.Ю., Ежлова Е.Б., Мельникова А.А., Башкетова Н.С., Фридман Р.К., Лялина Л.В., и др. Популяционный иммунитет к вирусу SARS-CoV-2 среди населения Санкт-Петербурга в период эпидемии COVID-19. Проблемы особо опасных инфекций. 2020; (3): 124–30. https://doi.org/10.21055/0370-1069-2020-3-124-130
- Расчёт необходимой численности выборки. Available at: https://bstudy.net/672834/sotsiologiya/raschet_neobhodimoy_chislennosti_vyborki (accessed August 12, 2021).
- Simon A.K., Hollander G.A., McMichael A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015; 282(1821): 20143085. https://doi.org/10.1098/rspb.2014.3085
- Wald A., Wolfowitz J. Confidence limits for continuous distribution functions. Ann. Math. Stat. 1939; 10(2): 105–18.
- Agresti A., Coull B.A. Approximate Is Better than “Exact” for Interval Estimation of Binomial Proportions. Am. Stat. 1998; 52(2): 119–26. https://doi.org/10.2307/2685469
- Исследовательская компания «РАДАР». Калькулятор значимых различий (z-test). Available at: https://radar-research.ru/software/z-test_calculator/ (accessed August 12, 2021).
- Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome Coronavirus 2. Emerg. Infect. Dis. 2020; 26(7): 1470–7. https://doi.org/10.3201/eid2607.200282
- Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y., et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J. Microbiol. Immunol. Infect. 2020; 53(3): 404–12. https://doi.org/10.1016/j.jmii.2020.02.012
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W., Shaman J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science. 2020; 368(6490): 489–93. https://doi.org/10.1126/science.abb3221
- Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARSCoV-2 infections. Nat. Med. 2020; 26(8): 1200–4. https://doi.org/10.1038/s41591-020-0965-6
- Попова А.Ю., Ежлова Е.Б., Мельникова А.А., Смирнов В.С., Лялина Л.В., Козловских Д.Н. Особенности серопревалентности к SARS-CoV-2 населения Среднего и Южного Урала в ранний период пандемии COVID-19. Эпидемиология и Вакцинопрофилактика. 2021; 20(3): 8–18. https://doi.org/10.31631/2073-3046-2021-20-3-8-18
- Попова А.Ю., Андреева Е.Е., Бабура Е.А., Балахонов С.В., Башкетова Н.С., Буланов М.В., и др. Особенности формирования серопревалентности населения Российской Федерации к нуклеокапсиду SARS-CoV-2 в первую волну эпидемии COVID-19. Инфекция и иммунитет. 2021; 11(2): 297–323. https://doi.org/10.15789/2220-7619-FOD-1684
- Попова А.Ю., Ежлова Е.Б., Мельникова А.А., Балахонов С.В., Чеснокова М.В., Дубровина В.И., и др. Опыт исследования серопревалентности к вирусу SARS-CoV-2 населения Иркутской области в период вспышки COVID-19. Проблемы особо опасных инфекций. 2020; (3): 106–13. https://doi.org/10.21055/0370-1069-2020-3-106-113
- Borges L.P., Martins A.F., de Melo M.S., de Oliveira M.G.B., de Rezende Neto J.M., Dósea M.B., et al. Seroprevalence of SARSCoV-2 IgM and IgG antibodies in an asymptomatic population in Sergipe, Brazil. Rev. Panam. Salud. Publica. 2020; 44: e108. https://doi.org/10.26633/RPSP.2020.108
- Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., et al. Dynamics and significance of the antibody response to SARS-CoV-2 infection. medRxiv. 2020; 5(52): 20155374. https://doi.org/10.1101/2020.07.18.20155374
- Wu F., Wang A., Liu M., Wang Q., Chen J., Xia S., et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020; 20047365. https://doi.org/https://doi.org/10.1101/2020.03.30.20047365
- Lei Q., Li Y., Hou H.Y., Wang F., Ouyang Z.Q., Zhang Y., et al. Antibody dynamics to SARS-CoV-2 in asymptomatic COVID-19 infections. Allergy. 2021; 76(2): 551–61. https://doi.org/10.1111/all.14622
- Schaeffer B., Taylor B., Bushman M., Hanage W.P. The devil in the details: Herd immunity and pandemic response. Cell Host Microbe. 2021; 27(7): 1048. https://doi.org/10.1016/j.chom.2021.06.017
- Vabret N. Antibody responses to SARS-CoV-2 short-lived. Nat. Rev. Immunol. 2020; 20(9): 519. https://doi.org/10.1038/s41577-020-0405-3
Supplementary files