Pre-clinical safety studies of intranasal virus-like particles based vaccine for prevention of COVID-19
- Authors: Chernoryzh Y.Y.1, Kondratieva V.M.1, Malkova А.P.2, Savochkina T.E.1, Eliseeva O.V.1, Latyshev O.E.1, Yakunin D.Y.1, Zaykova O.N.1, Sludnyakova E.S.1, Grebennikova T.V.1
-
Affiliations:
- N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
- Institute for Biomedical Research and Technology (IMBIIT)
- Issue: Vol 70, No 1 (2025)
- Pages: 35-46
- Section: ORIGINAL RESEARCHES
- URL: https://journal-vniispk.ru/0507-4088/article/view/287908
- DOI: https://doi.org/10.36233/0507-4088-278
- EDN: https://elibrary.ru/fzgyxe
- ID: 287908
Cite item
Abstract
Introduction. The large-scale and prolonged pandemic of the novel coronavirus disease (COVID-19) has demonstrated the need for effective vaccination. Along with immunogenicity, safety is a critical issue for vaccines, as public trust can contribute to the success or failure of immunization programs. In preclinical studies, we assessed the safety of an intranasal Virus-like particle (VLP)-based vaccine in mice and rats.
The aim of the study is to conduct preclinical acute and subchronic toxicity studies assessing local tolerability of an intranasal VLP vaccine against COVID-19 in accordance with good laboratory practice.
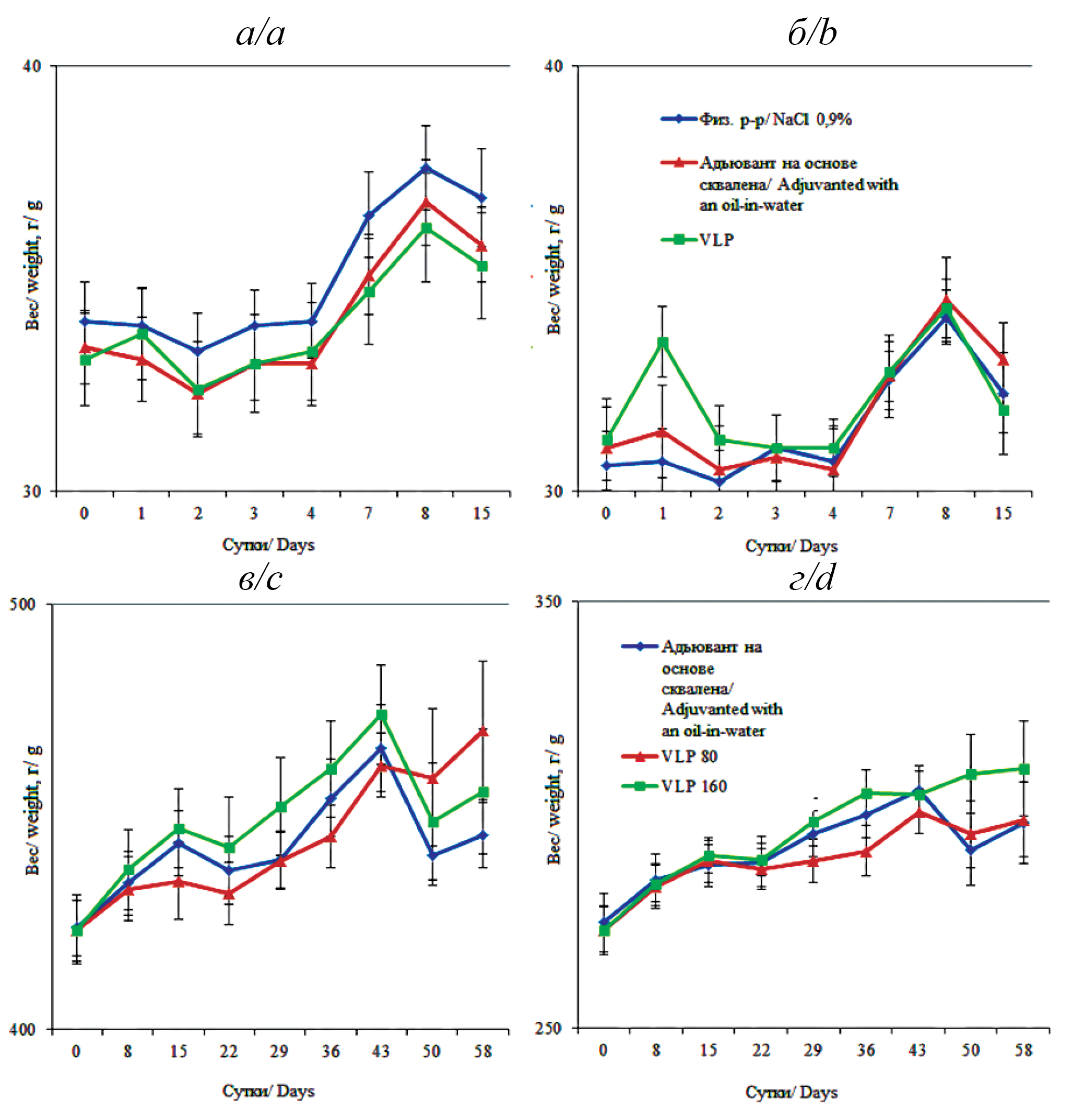
Materials and methods. Study was performed on adult outbreed mice (30 males, 30 females) and rats (45 males, 45 females). Physiological, morphometric and histological parameters, as well as general and biochemical blood tests and urine analysis were assessed.
Results. No deaths or intoxication were recorded in the acute toxicity study on mice, all parameters were within the physiological norm. In the subchronic toxicity study on rats, no changes in the general condition, behavior, or death of animals were noted. The structure of internal organs, blood and urine tests, hemostasis did not differ significantly between the groups. No local irritant effect was detected at the injection site during visual assessment, cytological and histological analysis.
Conclusion. The VLP vaccine is safe, as evidenced by the results of preclinical studies, does not negatively affect the function of various organs, the level of cellular and biochemical biomarkers in the blood and urine of mice and rats. Visual assessment, cytology and histology of the vaccine injection site did not reveal any local irritant effect.
Keywords
Full Text
##article.viewOnOriginalSite##About the authors
Yana Y. Chernoryzh
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Author for correspondence.
Email: revengeful_w@mail.ru
ORCID iD: 0000-0001-9848-8515
SPIN-code: 3576-8760
Scopus Author ID: 57203299151
ResearcherId: AAI-7206-2020
PhD, Candidate of Medical Sciences, Researcher, laboratory of molecular diagnostics
Russian Federation, 123098, MoscowValeria M. Kondratieva
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: 1999valeriak@mail.ru
ORCID iD: 0000-0001-9163-4516
graduate student, laboratory of molecular diagnostics
Russian Federation, 123098, MoscowАnastasia P. Malkova
Institute for Biomedical Research and Technology (IMBIIT)
Email: nastena0302@yandex.ru
ORCID iD: 0000-0002-2817-4817
head of the Laboratory of Biological Research
Russian Federation, 143090, Krasnoznamensk, Moscow regionTatyana E. Savochkina
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: tasavochkina@yandex.ru
ORCID iD: 0000-0003-4366-8476
Researcher, Laboratory of Molecular Diagnostics
Russian Federation, 123098, MoscowOlesya V. Eliseeva
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: olesenka80@mail.ru
ORCID iD: 0000-0002-0723-9749
PhD, Senior Scientist, Laboratory of molecular diagnostics
Russian Federation, 123098, MoscowOleg E. Latyshev
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: oleglat80@mail.ru
ORCID iD: 0000-0002-5757-3809
PhD, Senior Scientist, Laboratory of molecular diagnostics
Russian Federation, 123098, MoscowDmitriy Y. Yakunin
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: yd364@mail.ru
ORCID iD: 0009-0009-4531-5739
graduate student of laboratory of molecular diagnostics
Russian Federation, 123098, MoscowOlga N. Zaykova
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: zaykova_o_n@mail.ru
ORCID iD: 0000-0003-4708-2069
PhD, Senior Scientist at the Laboratory of Molecular Diagnostics
Russian Federation, 123098, MoscowEkaterina S. Sludnyakova
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: ekaterina.ses@mail.ru
ORCID iD: 0009-0000-4925-5205
Leading engineer for the implementation of scientific developments
Russian Federation, 123098, MoscowTatyana V. Grebennikova
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: t_grebennikova@mail.ru
ORCID iD: 0000-0002-6141-9361
Doctor of Biological Sciences, Professor, Corresponding Member RAS, deputy Director for Science of the Division of the Ivanovsky Virology Institute Head of the Control Center
Russian Federation, 123098, MoscowReferences
- Fernandes Q., Inchakalody V.P., Merhi M., Mestiri S., Taib N., Moustafa Abo El-Ella D., et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med. 2022; 54(1): 524–40. https://doi.org/10.1080/07853890.2022.2031274
- Grebennikova T.V., Eliseeva O.V., Latyshev O.E., Savochkina T.E., Tsibezov V.V., Cherepushkin S.A., et al. Recombinant virus-like particles for induction of specific immunity against severe acute respiratory syndrome virus SARS-CoV-2. Patent RU 2769224 C1; 2022. https://elibrary.ru/prmwjl (in Russian)
- Grebennikova T.V., Eliseeva O.V., Latyshev O.E., Cherepushkin S.A., Tsibizov V.V., Lebedeva V.V., et al. Virus-like chimeric particles for the induction of specific immunity against the severe acute respiratory syndrome virus SARS-CoV-2, containing proteins of coronavirus and rotavirus. Patent RU 2779810 C1; 2022. (in Russian)
- Gus’kova T.A., Syubaev R.D. Toxicological Aspects of Simultaneous Use of Various Medicines. Toxicology of Medicines [Toksikologicheskie aspekty odnovremennogo ispol’zovaniya razlichnykh lekarstvennykh sredstv. Toksikologiya lekarstvennykh sredstv]. Moscow: Russkii vrach; 2003; 116–40. (in Russian)
- Zapadnyuk I.P., Zapadnyuk V.I., Zakhariya E.A. Laboratory Animals, their Breeding and Use in Experiment [Laboratornye zhivotnye, ikh razvedenie i ispol’zovanie v eksperimente]. Kiev; 1982. (in Russian)
- International recommendations for conducting biomedical research using animals. Lanimagologiya. 1993; (1): 29. (in Russian)
- Menshikov V.V. Laboratory Research Methods in the Clinic [Laboratornye metody issledovaniya v klinike]. Moscow; 1987. (in Russian)
- Nazarenko G.I., Kishkun A.A. Clinical Evaluation of Laboratory Research Results: A Practical Guide [Klinicheskaya otsenka rezul’tatov laboratornykh issledovanii: Prakticheskoe rukovodstvo]. Moscow: Meditsina; 2007. (in Russian)
- Trakhtenberg I.M. Problems of Norm in Toxicology [Problemy normy v toksikologii]. Moscow; 1991. (in Russian)
- Mironov A.N. ed. Guidelines for Conducting Preclinical Studies of Medicines [Rukovodstvo po provedeniyu doklinicheskikh issledovanii lekarstvennykh sredstv]. Moscow: Grif and K; 2012. (in Russian)
- Mironov A.N., Merkulov V.A. Guidelines for the Examination of Medicines. Volume 3 [Rukovodstvo po ekspertize lekarstvennykh sredstv. Tom 3]. Moscow: Poligraf-Plyus; 2014. (in Russian)
- Garber D.S., Barbi R.V., Bilitski D.T., Kleiton Li.E., Donovan D.K., Kon D.F., et al. Guidelines for the Maintenance and Use of Laboratory Animals [Rukovodstvo po soderzhaniyu i ispol’zovaniyu laboratornykh zhivotnykh]. Moscow: IRBIS; 2017. https://elibrary.ru/zrjvdj (in Russian)
- WHO. Guidelines on the non-clinical evaluation of vaccine adjuvants and adjuvanted vaccines; 2014. Available at: https://who.int/publications/m/item/nonclinical-evaluation-of-vaccine-adjuvants-and-adjuvanted-vaccines-annex-2-trs-no-987
- Requirements of the International Committee for Science on the use of laboratory animals in experimental research. Byulleten’ IKLAS. 1978; (24): 4–5. (in Russian)
- Lineva A. Physiological Indicators of the Norm of Animals. Reference Book [Fiziologicheskie pokazateli normy zhivotnykh. Spravochnik]. Moscow: Aquarium LTD; 2003. (in Russian)
- Abrashova T.V., Gushchin Ya.A., Kovaleva M.A., Rybakova A.V., Selezneva A.I., Sokolova A.P., et al. Guide. Physiological, Biochemical and Biometric Indicators of the Norm of Experimental Animals [Spravochnik. Fiziologicheskie, biokhimicheskie i biometricheskie pokazateli normy eksperimental’nykh zhivotnykh]. St. Petersburg: LEMA; 2013. https://elibrary.ru/ptsruo (in Russian)
- Chen Y., Cheng L., Lian R., Song Z., Tian J. COVID-19 vaccine research focusses on safety, efficacy, immunoinformatics, and vaccine production and delivery: a bibliometric analysis based on VOSviewer. Biosci. Trends. 2021; 15(2): 64–73. https://doi.org/10.5582/bst.2021.01061
- Nooraei S., Bahrulolum H., Hoseini Z.S., Katalani C., Hajizade A., Easton A.J., et al. Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnology. 2021; 19(1): 59. https://doi.org/10.1186/s12951-021-00806-7
- Bai Z., Wan D., Lan T., Hong W., Dong H., Wei Y., et al. Nanoplatform based intranasal vaccines: current progress and clinical challenges. ACS Nano. 2024; 18(36): 24650–81. https://doi.org/10.1021/acsnano.3c10797
- Marks P.W., Gruppuso P.A., Adashi E.Y. Urgent need for next-generation COVID-19 vaccines. JAMA. 2023; 329(1): 19–20. https://doi.org/10.1001/jama.2022.22759
- Latyshev O.E., Zaykova O.N., Eliseeva O.V., Savochkina T.E., Chernoryzh Ya.Yu., Syroeshkin A.V., et al. Development, production and characterization of SARS-CoV-2 virus-like particles (Coronaviridae: orthocoronavirinae: betacoronavirus: sarbecovirus). Voprosy virusologii. 2024; 69(2): 175–86. https://doi.org/10.36233/0507-4088-226 https://elibrary.ru/gkxfed (in Russian)
- Banihashemi S.R., Es-Haghi A., Fallah Mehrabadi M.H., Nofeli M., Mokarram A.R., Ranjbar A., et al. Safety and efficacy of combined intramuscular/intranasal RAZI-COV PARS vaccine candidate against SARS-CoV-2: A preclinical study in several animal models. Front. Immunol. 2022; 13: 836745. https://doi.org/10.3389/fimmu.2022.836745
- Vakhrusheva A.V., Kudriavtsev A.V., Kryuchkov N.A., Deev R.V., Frolova M.E., Blagodatskikh K.A., et al. SARS-CoV-2 subunit virus-like vaccine demonstrates high safety profile and protective efficacy: preclinical study. Vaccines (Basel). 2022; 10(8): 1290. https://doi.org/10.3390/vaccines10081290
- Antonova N.A., Yeritsyan K.Yu. The systematic review of empirical research of factors of refusal from vaccination. Gigiena i Sanitaria (Hygiene and Sanitation, Russian journal). 2018; 97(7): 664–70. https://doi.org/10.18821/0016-9900-2018-97-7-664-670 https://elibrary.ru/uxaexo (in Russian)
Supplementary files