Дифференциальная диагностика хронических нарушений сознания по данным структурной магнитно-резонансной томографии
- Авторы: Сергеева А.Н.1, Морозова С.Н.1, Сергеев Д.В.1, Кремнева Е.И.1, Зимин А.А.1, Легостаева Л.А.1, Язева Е.Г.2, Кротенкова М.В.1, Рябинкина Ю.В.1, Супонева Н.А.1, Пирадов М.А.1
-
Учреждения:
- Научный центр неврологии
- ООО «Реабилитационный центр “Три сестры”»
- Выпуск: Том 5, № 2 (2024)
- Страницы: 190-202
- Раздел: Оригинальные исследования
- URL: https://journal-vniispk.ru/DD/article/view/264832
- DOI: https://doi.org/10.17816/DD569418
- ID: 264832
Цитировать
Аннотация
Обоснование. Дифференциальная диагностика хронических нарушений сознания остаётся сложной задачей даже для опытных клиницистов. В связи с этим для оценки таких пациентов актуальной является разработка инструментальных подходов, предоставляющих дополнительную информацию о диагнозе.
Цель — оценка межэкспертной согласованности и возможностей практического применения ранее предложенной шкалы оценки изменений на основе структурной магнитно-резонансной томографии для дифференциальной диагностики хронических нарушений сознания (DOC-MRIDS) на более крупной выборке пациентов.
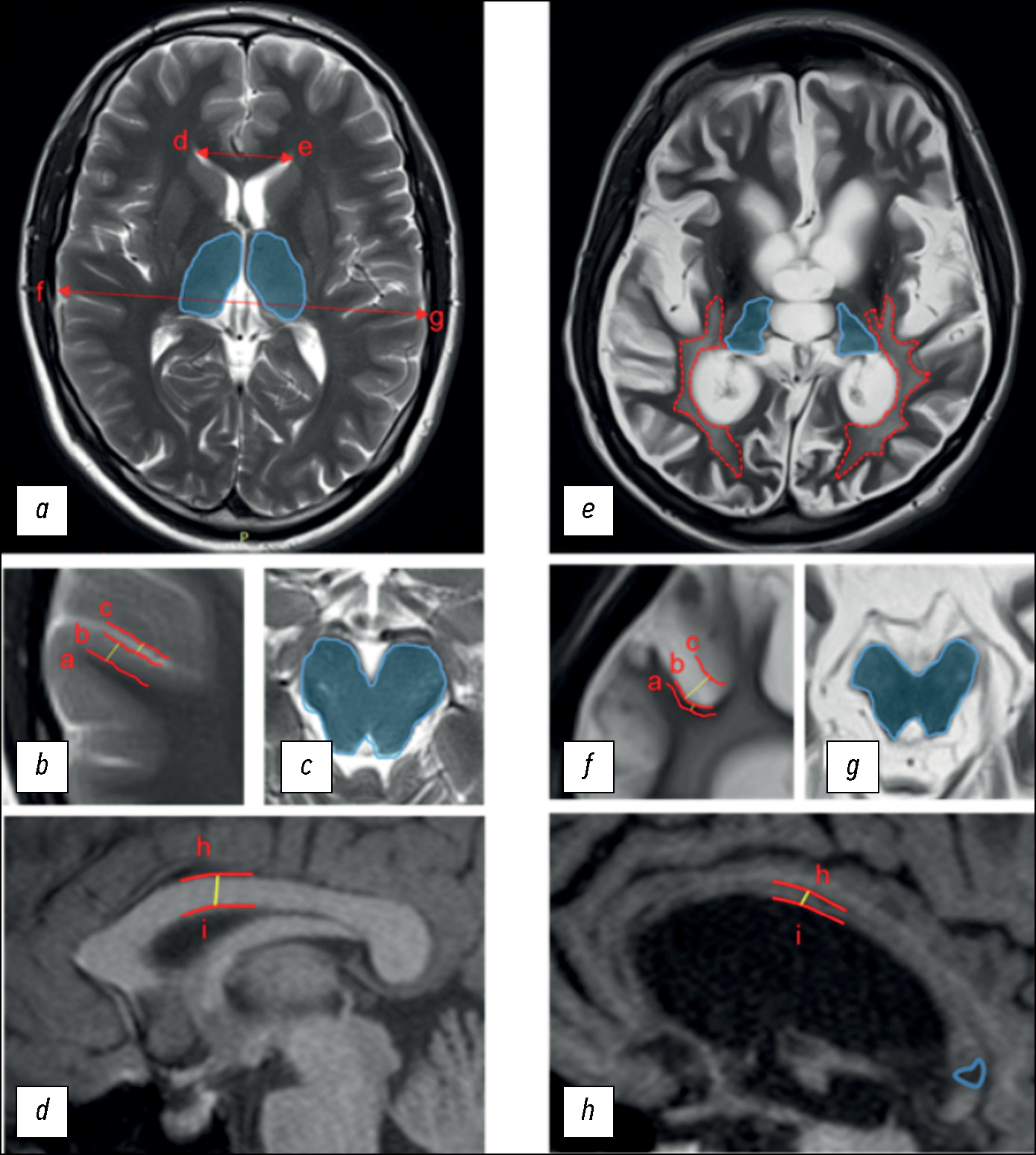
Материалы и методы. Исследованы 60 соматически стабильных пациентов с клинически диагностированными хроническими нарушениями сознания: 32 — в вегетативном состоянии, и 28 — в состоянии минимального сознания. Клиническая оценка проводилась с использованием пересмотренной шкалы восстановления после комы (CRS-R). Всем пациентам была проведена структурная магнитно-резонансная томография с использованием томографов 3.0 T Siemens, включающая T2- и T1-последовательности. При оценке структурных изменений по шкале DOC-MRIDS учитывались наличие и выраженность следующих признаков: диффузная атрофия коры, увеличение желудочков, расширение борозд, лейкоареоз, дегенерация ствола мозга и/или таламуса, дегенерация мозолистого тела, очаговое поражение мозолистого тела; производился подсчёт суммарного балла. Данные магнитно-резонансной томографии анализировались тремя нейрорадиологами с оценкой межэкспертной согласованности (коэффициент альфа Криппендорфа).
Результаты. Выявлена высокая межэкспертная согласованность оценки по шкале DOC-MRIDS: α=0,806 (95% доверительный интервал 0,757–0,849). Пациенты в вегетативном состоянии имели более высокий балл по шкале магнитно-резонансной томографии DOC-MRIDS по сравнению с пациентами в состоянии минимального сознания (p <0,005). Получена отрицательная корреляция между оценкой по шкалам CRS-R и DOC-MRIDS (ρ=–0,457, p <0,0001) между отдельными доменами клинической шкалы и признаками по магнитно-резонансной томографии.
Заключение. Оценка структурных изменений у пациентов с хроническими нарушениями сознания с помощью шкалы DOC-MRIDS помогает установить вероятный клинический тип нарушения сознания с достаточной специфичностью, чувствительностью и межэкспертной согласованностью и может использоваться в клинической практике как дополнительный к клиническим данным дифференциально-диагностический метод.
Полный текст
Открыть статью на сайте журналаОб авторах
Анастасия Николаевна Сергеева
Научный центр неврологии
Автор, ответственный за переписку.
Email: sergeeva@neurology.ru
ORCID iD: 0000-0002-2481-4565
SPIN-код: 6761-8250
канд. мед. наук
Россия, МоскваСофья Николаевна Морозова
Научный центр неврологии
Email: kulikovasn@gmail.com
ORCID iD: 0000-0002-9093-344X
SPIN-код: 2434-7827
канд. мед. наук
Россия, МоскваДмитрий Владимирович Сергеев
Научный центр неврологии
Email: dmsergeev@yandex.ru
ORCID iD: 0000-0002-9130-1292
SPIN-код: 8282-3920
канд. мед. наук
Россия, МоскваЕлена Игоревна Кремнева
Научный центр неврологии
Email: moomin10j@mail.ru
ORCID iD: 0000-0001-9396-6063
SPIN-код: 8799-8092
канд. мед. наук
Россия, МоскваАлексей Алексеевич Зимин
Научный центр неврологии
Email: leha-zimin@inbox.ru
ORCID iD: 0000-0002-9226-2870
SPIN-код: 9525-1805
Россия, Москва
Людмила Александровна Легостаева
Научный центр неврологии
Email: milalegostaeva@gmail.com
канд. мед. наук
Россия, МоскваЕлизавета Григорьевна Язева
ООО «Реабилитационный центр “Три сестры”»
Email: lizaveta.mochalova@gmail.com
ORCID iD: 0000-0003-0382-7719
SPIN-код: 4895-3900
канд. мед. наук
Россия, МоскваМарина Викторовна Кротенкова
Научный центр неврологии
Email: krotenkova_mrt@mail.ru
ORCID iD: 0000-0003-3820-4554
SPIN-код: 9663-8828
д-р мед. наук
Россия, МоскваЮлия Валерьевна Рябинкина
Научный центр неврологии
Email: ryabinkina11@mail.ru
ORCID iD: 0000-0001-8576-9983
SPIN-код: 5044-2701
д-р мед. наук
Россия, МоскваНаталья Александровна Супонева
Научный центр неврологии
Email: nasu2709@mail.ru
ORCID iD: 0000-0003-3956-6362
SPIN-код: 3223-6006
д-р мед. наук, член-корреспондент РАН, профессор
Россия, МоскваМихаил Александрович Пирадов
Научный центр неврологии
Email: mpi711@gmail.com
ORCID iD: 0000-0002-6338-0392
SPIN-код: 2860-1689
д-р мед. наук, академик РАН, профессор
Россия, МоскваСписок литературы
- Koch C., Massimini M., Boly M., Tononi G. Neural correlates of consciousness: progress and problems // Nat Rev Neurosci. 2016. Vol. 17, N 5. P. 307–321. doi: 10.1038/nrn.2016.22
- Monti M.M., Laureys S., Owen A.M. The vegetative state // BMJ. 2010. Vol. 341. P. 376–385. doi: 10.1136/bmj.c3765
- Giacino J.T., Ashwal S., Childs N., et al. The minimally conscious state: Definition and diagnostic criteria // Neurology. 2002. Vol. 58, N 3. Р. 349–353. doi: 10.1212/wnl.58.3.349
- Белкин А.А., Александрова Е.В., Ахутина Т.В., и др. Хронические нарушения сознания: клинические рекомендации Общероссийской общественной организации «Федерация анестезиологов и реаниматологов» // Вестник интенсивной терапии имени А.И. Салтанова. 2023. № 3. C. 7–42. doi: 10.21320/1818-474X-2023-3-7-42
- Giacino J.T. The vegetative and minimally conscious states: Consensus-based criteria for establishing diagnosis and prognosis // Neurorehabilitation. 2004. Vol. 19, N 4. P. 293–298. doi: 10.3233/NRE-2004-19405
- Seel R.T., Sherer M., et al. Assessment scales for disorders of consciousness: evidence-based recommendations for clinical practice and research // Arch Phys Med Rehabil. 2010. Vol. 91, N 12. P. 1795–1813. doi: 10.1016/j.apmr.2010.07.218
- Schnakers C., Vanhaudenhuyse A., Giacino J., et al. Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment // BMC Neurol. 2009. N 9. P. 35–40. doi: 10.1186/1471-2377-9-35
- Stender J., Gosseries O., Bruno M., et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: A clinical validation study // Lancet. 2014. Vol. 384, N 9942. P. 514–522. doi: 10.1016/S0140-6736(14)60042-8
- Monti M., Vanhaudenhuyse A., Coleman M., et. al. Willful modulation of brain activity in disorders of consciousness // N. Engl. J. Med. 2010. Vol. 362, N 7. P. 579–589. doi: 10.1056/NEJMoa0905370
- Crone J., Bio B., Vespa P., et. al. Restoration of thalamo-cortical connectivity after brain injury: Recovery of consciousness, complex behavior, or passage of time // J. Neurosci. Res. 2018. Vol. 96, N 4. P. 671–687. doi: 10.1002/jnr.24115
- Demertzi A., Antonopoulos G., Heine L., et al. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients // Brain. 2015. Vol. 138, N 9. P. 2619–2631. doi: 10.1093/brain/awv169
- Lutkenhoff E., Chiang J., Tshibanda L., et al. Thalamic and extrathalamic mechanisms of consciousness after severe brain injury // Ann. Neurol. 2015. Vol. 78, N 1. P. 68–76. doi: 10.1002/ana.24423
- Guldenmund P., Soddu A., Baquero K., et al. Structural brain injury in patients with disorders of consciousness: A voxel-based morphometry study // Brain Inj. 2016. Vol. 30, N 3. P. 343–352. doi: 10.3109/02699052.2015.1118765
- Annen J., Frasso G., Crone J., et al. Regional brain volumetry and brain function in severely brain-injured patients // Ann. Neurol. 2018. Vol. 83, N 4. P. 842–853. doi: 10.1002/ana.25214
- Morozova S.N., Kremneva E.I., Sergeev D.V., et al. Conventional Structural Magnetic Resonance Imaging in Differentiating Chronic Disorders of Consciousness // Brain Sci. 2018. Vol. 8, N 8. P. 144–155. doi: 10.3390/brainsci8080144
- Легостаева Л.А., Мочалова Е.Г., Супонева Н.А., и др. Сложности клинической диагностики хронических нарушений сознания и рекомендации по клинико-инструментальной оценке пациентов после их выхода из комы // Анестезиология и реаниматология. 2017. Т. 62, № 6. C. 449–456. EDN: YPLNJY doi: 10.18821/0201-7563-2017-62-6-449-456
- Соловьева П.И., Синкин М.В., Талыпов А.Э., и др. Клиническая оценка пациентов с хроническим нарушением сознания врачами разных специальностей // Анналы клинической и экспериментальной неврологии. 2022. Т. 16, № 2. C. 44–49. doi: 10.54101/ACEN.2022.2.5
- Medina J.P., Nigri A., Stanziano M., et al. Resting-State fMRI in Chronic Patients with Disorders of Consciousness: The Role of Lower-Order Networks for Clinical Assessment // Brain Sci. 2022. Vol. 12, N 3. P. 355–374. doi: 10.3390/brainsci12030355
- Rohaut B., Doyle K.W., Reynolds A.S., et al. Deep structural brain lesions associated with consciousness impairment early after hemorrhagic stroke // Sci Rep. 2019. Vol. 9, N 1. P. 4174. doi: 10.1038/s41598-019-41042-2
- Alnagger N., Cardone P., Martial C., et al. The current and future contribution of neuroimaging to the understanding of disorders of consciousness // Presse Med. 2023. Vol. 52, N 2. P. 104163. doi: 10.1016/j.lpm.2022.104163
- Бакулин И.С., Кремнева Е.И., Кузнецов А.В., и др. Хронические нарушения сознания / под ред. М.А. Пирадова. Москва : Горячая линия-Телеком, 2020.
Дополнительные файлы