Remote monitoring of patients with chronic heart failure: a non invasive approach
- Autores: Emelianov A.V.1, Kozhevnikova M.V.1, Zheleznykh E.A.1, Panova A.L.1, Privalova E.V.1, Belenkov Y.N.1
-
Afiliações:
- Sechenov First Moscow State Medical University
- Edição: Volume 5, Nº 4 (2024)
- Páginas: 794-807
- Seção: Reviews
- URL: https://journal-vniispk.ru/DD/article/view/309837
- DOI: https://doi.org/10.17816/DD633033
- ID: 309837
Citar
Resumo
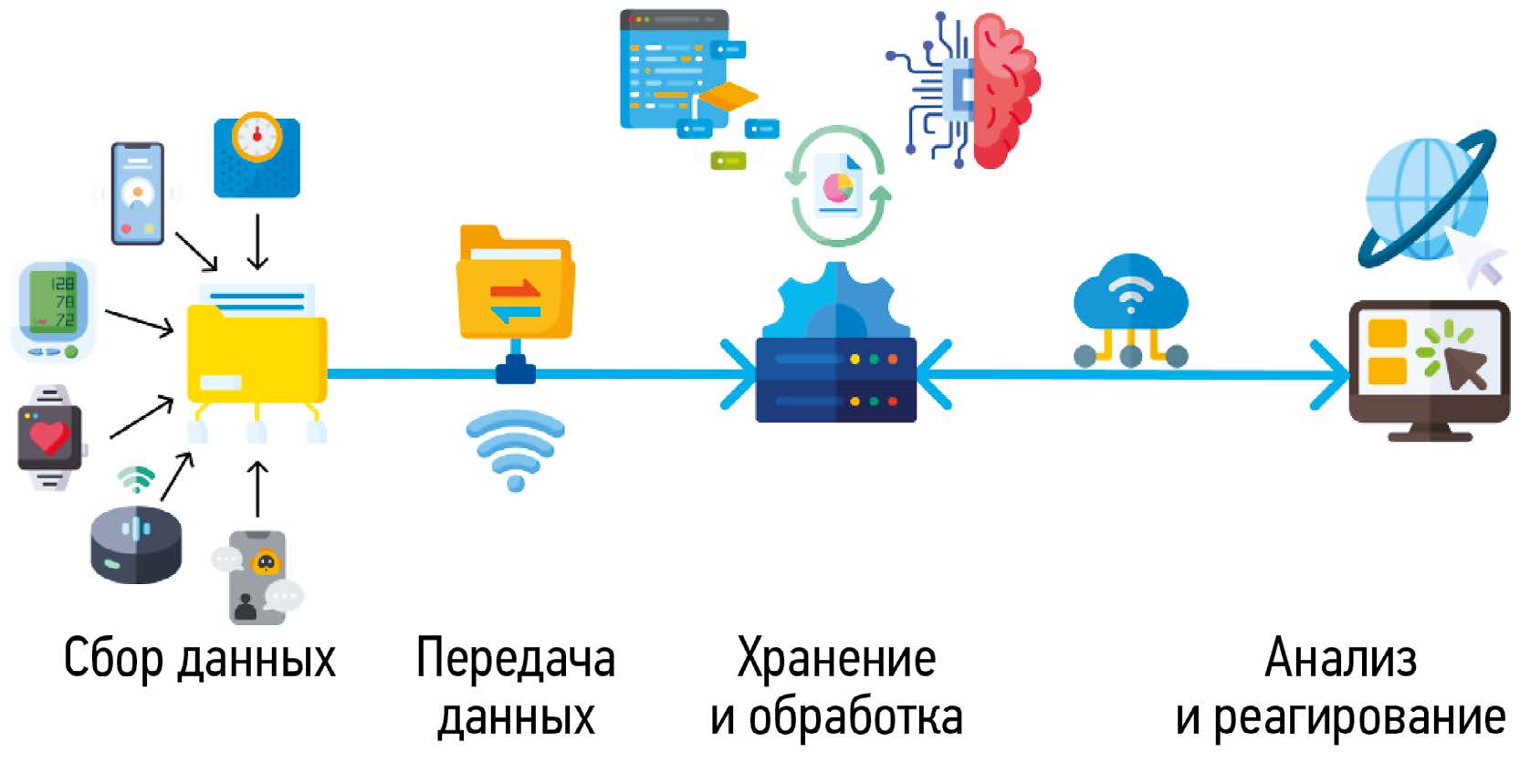
Remote monitoring of patients, including those with chronic heart failure, has been actively used in recent years. Unlike invasive methods, non invasive methods are not associated with surgical risks and offer a wide range of patient management options such as telemonitoring, virtual visits, emergency department pre triage, in hospital telemedicine, telemedicine rehabilitation, psychological support, etc. Previously, remote monitoring required a multidisciplinary medical team to ensure high efficiency, and attempts to use advanced technology to reduce human involvement were often unsuccessful. However, all electronic and telemedicine technologies in healthcare have been dramatically transformed by the COVID-19 pandemic. There is currently a wide variety of remote monitoring methods and technologies. But it is still impossible to clearly assess their effectiveness due to a lack of common standards, inadequate legislation, and regional, social, and economic differences in the availability of these technologies. However, in 2021, remote monitoring was included in the European Society of Cardiology clinical guidelines for the diagnosis and management of acute and chronic heart failure (IIb). This review describes the history of modern remote monitoring methods and the problems they are designed to solve in order to improve outpatient health monitoring for patients with chronic heart failure.
Palavras-chave
Texto integral
##article.viewOnOriginalSite##Sobre autores
Aleksei Emelianov
Sechenov First Moscow State Medical University
Autor responsável pela correspondência
Email: emelyanow.alexei@yandex.ru
ORCID ID: 0000-0002-4748-8029
Código SPIN: 4346-1475
MD
Rússia, MoscowMaria Kozhevnikova
Sechenov First Moscow State Medical University
Email: kozhevnikova-m@inbox.ru
ORCID ID: 0000-0003-4778-7755
Código SPIN: 8501-9812
MD, Cand. Sci. (Medicine)
Rússia, MoscowElena Zheleznykh
Sechenov First Moscow State Medical University
Email: elenavlvp@gmail.com
ORCID ID: 0000-0002-2596-192X
Código SPIN: 2941-4875
MD, Cand. Sci. (Medicine)
Rússia, MoscowAnastasia Panova
Sechenov First Moscow State Medical University
Email: lanaer@rambler.ru
ORCID ID: 0009-0000-9543-5282
MD
Rússia, MoscowElena Privalova
Sechenov First Moscow State Medical University
Email: ev_privalova@mail.ru
ORCID ID: 0000-0001-6675-7557
MD, Dr. Sci. (Medicine), Professor
Rússia, MoscowYuri Belenkov
Sechenov First Moscow State Medical University
Email: ynbelenkov@gmail.com
ORCID ID: 0000-0002-3014-6129
Código SPIN: 5661-4691
MD, Dr. Sci. (Medicine), Academician of the Russian Academy of Sciences, head of the department of hospital therapy №1
Rússia, MoscowBibliografia
- McDonagh TA, Metra M, Adamo M, et al.; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368
- Polyakov DS, Fomin IV, Belenkov YuN, et al. Chronic heart failure in the Russian Federation: what has changed over 20 years of follow up? Results of the EPOCH-CHF study. Kardiologiia. 2021;61(4):4–14. EDN: WSZNFS doi: 10.18087/cardio.2021.4.n1628
- Tromp J, Ouwerkerk W, van Veldhuisen DJ, et al. A Systematic review and network meta analysis of pharmacological treatment of heart failure with reduced ejection fraction // JACC Heart Fail. 2022;10(2):73–84. doi: 10.1016/j.jchf.2021.09.004
- Shlyakhto EV, Belenkov YuN, Boytsov SA, et al. Prospective observational multicenter registry study of patients with heart failure in the Russian Federation (PRIORITET-CHF): rationale, objectives and design of the. Russian Journal of Cardiology. 2023;28(6):7–14. EDN: LKSHVP doi: 10.15829/1560-4071-2023-5456
- Maddox TM, Januzzi JL, Jr, Allen LA, et al. 2024 ACC Expert consensus decision pathway for treatment of heart failure with reduced ejection fraction: a report of the American College of Cardiology solution set oversight committee. J. Am. Coll. Cardiol. 2024;83(15):1444–1488. doi: 10.1016/j.jacc.2023.12.024
- Ruppar TM, Cooper PS, Mehr DR, et al. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta analysis of controlled trials. J. Am. Heart Assoc. 2016;5(6):e002606. doi: 10.1161/JAHA.115.002606
- Wu JR, Moser DK. Medication adherence mediates the relationship between heart failure symptoms and cardiac event free survival in patients with heart failure. J. Cardiovasc. Nurs. 2018;33(1):40–46. doi: 10.1097/JCN.0000000000000427
- Shah D, Simms K, Barksdale D, Wu J. Improving medication adherence of patients with chronic heart failure: challenges and solutions. Res. Rep. Clin. Cardiol. 2015;6:87–95. doi: 10.2147/RRCC.S50658
- Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr. Heart Fail. Rep. 2009;6(4):287–292. doi: 10.1007/s11897-009-0039-z
- Stevenson LW, Ross HJ, Rathman LD, Boehmer JP. Remote monitoring for heart failure management at home. J. Am. Coll. Cardiol. 2023;81(23):2272–2291. doi: 10.1016/j.jacc.2023.04.010
- Lindenfeld J, Costanzo MR, Zile MR, et al.; GUIDE-HF, CHAMPION, LAPTOP-HF Investigators. Implantable hemodynamic monitors improve survival in patients with heart failure and reduced ejection fraction. J. Am. Coll. Cardiol. 2024;83(6):682–694. doi: 10.1016/j.jacc.2023.11.030
- Scholte NTB, Gürgöze MT, Aydin D, et al. Telemonitoring for heart failure: a meta analysis. Eur. Heart J. 2023;44(31):2911–2926. doi: 10.1093/eurheartj/ehad280
- Rich MW, Beckham V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N. Engl. J. Med. 1995;333(18):1190–1195. doi: 10.1056/NEJM199511023331806
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J. Am. Coll. Cardiol. 1997;30(3):725–732. doi: 10.1016/s0735-1097(97)00208 8
- Cline CM, Israelsson BY, Willenheimer RB, et al. Cost effective management programme for heart failure reduces hospitalization. Heart. 1998;80(5):442–446. doi: 10.1136/hrt.80.5.442
- Gattis WA, Hasselblad V, Whellan DJ, O’Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the pharmacist in heart failure assessment recommendation and monitoring (PHARM) Study. Arch. Intern. Med. 1999;159(16):1939–1945. doi: 10.1001/archinte.159.16.1939
- McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission. J. Am. Coll. Cardiol. 2004;44(4):810–819. doi: 10.1016/j.jacc.2004.05.055
- Cleland JG, Louis AA, Rigby AS, et al.; TEN-HMS Investigators. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death. J. Am. Coll. Cardiol. 2005;45(10):1654–1664. doi: 10.1016/j.jacc.2005.01.050
- Belenkov YuN. Effect of specialized forms of active outpatient management on the functional status, quality of life and hemodynamic parameters in patients with advanced heart failure. Results of the Russian Program «CHANCE». Russ Heart Fail J. 2007;8(3):112–116. EDN: ODSNQP
- Rotenstein LS, Holmgren AJ, Downing N L, Bates DW. Differences in total and after hours electronic health record time across ambulatory specialties. JAMA Intern. Med. 2021;181(6):863–865. doi: 10.1001/jamainternmed.2021.0256
- Adler Milstein J, Zhao W, Willard Grace R, et al. Electronic health records and burnout: time spent on the electronic health record after hours and message volume associated with exhaustion but not with cynicism among primary care clinicians. J. Am. Med. Inform. Assoc. 2020;27(4):531–538. doi: 10.1093/jamia/ocz220
- Azizi Z, Broadwin C, Islam S, et al. Digital health interventions for heart failure management in underserved rural areas of the united states: a systematic review of randomized trials. J. Am. Heart Assoc. 2024;13(2):e030956. doi: 10.1161/JAHA.123.030956
- Klersy C, De Silvestri A, Gabutti G, et al. A meta analysis of remote monitoring of heart failure patients. J. Am. Coll. Cardiol. 2009;54(18):1683–1694. doi: 10.1016/j.jacc.2009.08.017
- Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in Patients with Heart Failure. N. Engl. J. Med. 2010;363(24):2301–2309. doi: 10.1056/NEJMoa1010029
- Koehler F, Winkler S, Schieber M, et al.; TIM-HF Investigators. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123(17):1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473
- Lyngå P, Persson H, Hägg Martinell A, et al. Weight monitoring in patients with severe heart failure (WISH). A randomized controlled trial. Eur. J. Heart Fail. 2012;14(4):438–444. doi: 10.1093/eurjhf/hfs023
- Boyne JJ, Vrijhoef HJ, Crijns HJ, et al.; TEHAF investigators. Tailored telemonitoring in patients with heart failure: results of a multicentre randomized controlled trial. Eur. J. Heart Fail. 2012;14(7):791–801. doi: 10.1093/eurjhf/hfs058
- Dendal P, De Keulenaer G, Troisfontaines P, et al. Effect of a telemonitoring facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: the TEMA-HF 1 (TElemonitoring in the MAnagement of Heart Failure) study. Eur. J. Heart Fail. 2012;14(3):333–340. doi: 10.1093/eurjhf/hfr144
- Inglis SC, Clark RA, Dierckx R, et al. Structured telephone support or non invasive telemonitoring for patients with heart failure. Cochrane Database Syst. Rev. 2015;2015(10):CD007228. doi: 10.1002/14651858.CD007228.pub3
- Cowie MR, Bax J, Bruining N, et al. e-Health: a position statement of the European Society of Cardiology. Eur. Heart J. 2016;37(1):63–66. doi: 10.1093/eurheartj/ehv416
- Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition–heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern. Med. 2016;176(3):310–318. doi: 10.1001/jamainternmed.2015.7712
- Grebennikova AA, Stoliarov AU, Lopatin YuM. The use of platform for remote monitoring on the base of mobile app for improving self-care in patients with chronic heart failure. Kardiologiia. 2017;57(4S):11–18. EDN: YKUOWF doi: 10.18087/cardio.2413
- Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel group, unmasked trial. The Lancet. 2018;392(10152):1047–1057. doi: 10.1016/S0140-6736(18)31880-4
- Mareev YuV, Zinchenko AO, Myasnikov RP, et al. Telemonitoring in patients with chronic heart failure. Kardiologiia. 2019;59(9S):4–15. EDN: ISWIAY doi: 10.18087/cardio.n530
- Zhu Y, Gu X, Xu C Effectiveness of telemedicine systems for adults with heart failure: a meta analysis of randomized controlled trials. Heart Fail. Rev. 2020;25(2):231–243. doi: 10.1007/s10741-019-09801-5
- Tersalvi G, Winterton D, Cioffi GM, et al. Telemedicine in heart failure during COVID-19: a step into the future. Front. Cardiovasc. Med. 2020;7:612818. doi: 10.3389/fcvm.2020.612818
- Nasonova SN, Lapteva AE, Zhirov IV, et al. Remote monitoring of patients with heart failure in real clinical practice. Kardiologiia. 2021;61(8):76–86. (In Russ.) EDN: GRIBYY doi: 10.18087/cardio.2021.8.n1683
- Yokota T, Fukushima A, Tsuchihashi Makaya M, et al. The AppCare-HF randomized clinical trial: a feasibility study of a novel self-care support mobile app for individuals with chronic heart failure. Eur. Heart J. - Digit. Health. 2023;4(4):325–336. doi: 10.1093/ehjdh/ztad032
- Rao VN, Kaltenbach LA, Granger BB, et al. The Association of Digital Health Application use with heart failure care and outcomes: insights from CONNECT-HF. J. Card. Fail. 2022;28(10):1487–1496. doi: 10.1016/j.cardfail.2022.07.050
- Agapov VV, Kudryashov YuY, Graifer IV, Samitin VV. Development and implementation of a heart failure telemonitoring system: the single centre experience. Kardiologiia. 2022;62(5):45–52. EDN: BHAITY doi: 10.18087/cardio.2022.5.n1825
- Radhakrishnan K, Julien C, O’Hair M, et al. Sensor controlled digital game for heart failure self management: protocol for a randomized controlled trial. JMIR Res. Protoc. 2023;12:e45801. doi: 10.2196/45801
- Lukey A, Mackay M, Hasan K, Rush KL. Clinical perspectives on the development of a gamified heart failure patient education web site. CIN Comput. Inform. Nurs. 2023;41(8):615–620. doi: 10.1097/CIN.0000000000000983
- Yun S, Enjuanes C, Cobo M, et al. Effect on cardiovascular mortality and worsening heart failure of mHealth solutions combining telemonitoring and teleintervention: results of the HERMeS multicentre, randomised, controlled trial. In: Heart Failure; 2023 May 20–23; Prague. Unpublished.
- Shara N, Bjarnadottir MV, Falah N, et al. Voice activated remote monitoring technology for heart failure patients: Study design, feasibility and observations from a pilot randomized control trial. PLOS ONE. 2022;17(5):e0267794. doi: 10.1371/journal.pone.0267794
- Emelianov AV, Zheleznykh EA, Kozhevnikova MV, et al. Quality of life and adherence to therapy in patients with chronic heart failure who were remotely monitored by chatbot compared to the standard follow-up group for 3 months. Digital Diagnostics. 2023;4(1S):53–56. EDN: PPGJTP doi: 10.17816/DD430343
- Pyrikova NV, Mozgunov NA, Osipova IV. Results of pilot remote monitoring of heart failure patients. Cardiovascular Therapy and Prevention. 2022;21(6):42–51. EDN: ROTHHY doi: 10.15829/1728-8800-2022-3151
- Wohlfahrt P, Jenča D, Melenovský V, et al. Remote heart failure symptoms assessment after myocardial infarction identifies patients at risk for death. J. Am. Heart Assoc. 2024;13(2):e032505. doi: 10.1161/JAHA.123.032505
- Stehlik J, Schmalfuss C, Bozkurt B, et al. Continuous wearable monitoring analytics predict heart failure hospitalization: the LINK-HF multicenter study. Circ. Heart Fail. 2020;13(3):e006513. doi: 10.1161/CIRCHEARTFAILURE.119.006513
- Potapov AP, Yartsev SE, Lagutova EA. Remote monitoring of patients with chronic heart failure using blood pressure telemonitoring and ECG. Russian Journal of Telemedicine and E-Health. 2021;7(3):42–51. EDN: OFOTQN doi: 10.29188/2712-9217-2021-7-3-42-51
- Garanin AA, Mullova IS, Shkaeva OV, et al. Remote monitoring of outpatients discharged from the emergency cardiac care department. Russian Journal of Cardiology. 2022;27(3S):8–15. EDN: BIRQPJ doi: 10.15829/1560-4071-2022-5072
- Kleiner Shochat M, Fudim M, Shotan A, et al. Prediction of readmissions and mortality in patients with heart failure: lessons from the IMPEDANCE-HF extended trial. ESC Heart Fail. 2018;5(5):788–799. doi: 10.1002/ehf2.12330
- Abraham WT, Anker S, Burkhoff D, et al. Primary results of the sensible medical innovations lung fluid status monitor allows reducing readmission rate of heart failure patients (smile) trial. J. Card. Fail. 2019;25(11):938. doi: 10.1016/j.cardfail.2019.11.007
- Bensimhon D, Alali SA, Curran L, et al. The use of the reds noninvasive lung fluid monitoring system to assess readiness for discharge in patients hospitalized with acute heart failure: A pilot study. Heart Lung. 2021;50(1):59–64. doi: 10.1016/j.hrtlng.2020.07.003
- Murton OM, Dec GW, Hillman RE, et al. Acoustic voice and speech biomarkers of treatment status during hospitalization for acute decompensated heart failure. Appl. Sci. 2023;13(3):1827. doi: 10.3390/app13031827
- Schöbi D, Zhang YP, Kehl J, et al. Evaluation of speech and pause alterations in patients with acute and chronic heart failure. J. Am. Heart Assoc. 2022;11(21):e027023. doi: 10.1161/JAHA.122.027023
- Atluri N, Mishra SR, Anderson T, et al. Acceptability of a text message based mobile health intervention to promote physical activity in cardiac rehabilitation enrollees: a qualitative substudy of participant perspectives. J. Am. Heart Assoc. 2024;13(2):e030807. doi: 10.1161/JAHA.123.030807
- Rossetto F, Borgnis F, Isernia S, et al. System integrated digital empowering and telerehabilitation to promote patient activation and well being in chronic disabilities: a usability and acceptability study. Front. Public Health. 2023;11:1154481. doi: 10.3389/fpubh.2023.1154481
- Piotrowicz E, Pencina MJ, Opolski G, et al. Effects of a 9 week hybrid comprehensive telerehabilitation program on long term outcomes in patients with heart failure: the telerehabilitation in heart failure patients (TELEREH-HF) randomized clinical trial. JAMA Cardiol. 2020;5(3):300–308. doi: 10.1001/jamacardio.2019.5006
- Dinesen B, Hansen ET, Refsgaard J, et al. «Future patient II» telerehabilitation for patients with heart failure: protocl for a randomized controlled trial. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024;20:200239. doi: 10.1016/j.ijcrp.2024.200239
- Ghazi L, Yamamoto Y, Fuery M, et al. Electronic health record alerts for management of heart failure with reduced ejection fraction in hospitalized patients: the PROMPT-AHF trial. Eur. Heart J. 2023;44(40):4233–4242. doi: 10.1093/eurheartj/ehad512
- Day S, Shah V, Kaganoff S, et al. Assessing the clinical robustness of digital health startups: cross sectional observational analysis. J. Med. Internet Res. 2022;24(6):e37677. doi: 10.2196/37677
Arquivos suplementares